GMP Proteins

🧪 BMP2-123H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
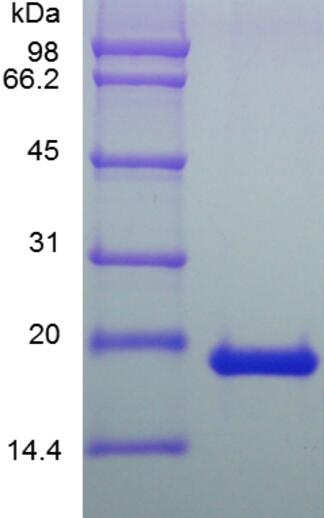
🧪 IL18-153HG
Source: HEK293
Species: Human
Tag:
Conjugation:
Protein Length: Tyr37-Asp193

🧪 IL11-4326HG
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:

🧪 LIF-4343HG
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
🧪 IL11-101HG
Source: Insect Cells
Species: Human
Tag: Non
Conjugation:
Protein Length: 22-199 a.a.

🧪 CSF1-110HG
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 33-190 a.a.

🧪 Mstn-121HG
Source: Mammalian Cells
Species: Human
Tag: Non
Conjugation:
Protein Length: 268-376 a.a.

🧪 IL25-136HG
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 33-177 a.a.

🧪 GDF11-137HG
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 299-407 a.a.

🧪 HGF-143HG
Source: Mammalian Cells
Species: Human
Tag: Non
Conjugation:
Protein Length: 32-728 a.a.

🧪 HPN-147HG
Source: Mammalian Cells
Species: Human
Tag:
Conjugation:
Protein Length: 45-417 a.a.

🧪 IL12-158HG
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length: 23-219;23-328 a.a.

Background
Overview
GMP-grade proteins refer to protein products produced under the conditions of Good Manufacturing Practice (GMP). Specifically, GMP is a set of widely recognized manufacturing and quality control standards that aim to ensure the safety and effectiveness of pharmaceutical products for their intended use. GMP covers the entire pharmaceutical process from raw material procurement, production process, finished product inspection to product release, requiring enterprises to establish and maintain a strict quality management system. For GMP-grade proteins, these protein products are typically used in the preclinical and clinical research phase and are manufactured under independent quality assurance (QA) supervision to ensure product quality and consistency. Each batch of GMP-grade recombinant protein is subjected to a series of rigorous quality control (QC) tests, including but not limited to bioactivity tests, to verify its quality and performance.

Differences Between non-GMP-Grade proteins
The difference between GMP-grade proteins and non-GMP-grade proteins are mainly reflected in the strict degree of quality management and production process. The details are as follows:
- Quality management system: GMP grade protein production requires that enterprises must establish and maintain a strict quality management system to ensure that every step of the product from raw material procurement, production process to finished product inspection meets GMP standards. Non-gmp grade proteins may not have such a strict quality control system.
- Production process control: The production of GMP grade proteins needs to be carried out in a strictly monitored environment to ensure product consistency and traceability. Non-gmp grade proteins may not require as much environmental control during production.
- Selection of raw materials: In the production of GMP grade proteins, the materials used need to comply with GMP standards, which usually means higher quality requirements. Non-gmp grade proteins may use raw materials with lower quality levels.
- Product release criteria: GMP grade proteins are subject to a series of quality control tests prior to release to ensure their safety and effectiveness. The release criteria for non-GMP grade proteins may be less stringent.

Production Process
The production process for GMP-grade proteins is a rigorous and meticulous process that involves multiple steps from genetic engineering to final product release. Specifically include the following links:
Codon optimization and gene synthesis: This is the initial stage of the production process, where the gene sequence of the target protein is optimized through genetic engineering techniques to improve the efficiency of expression in a specific expression system.
Strain construction: The optimized gene is inserted into a suitable host cell to build a strain that can efficiently express the target protein.
Process development: Develop fermentation or cell culture processes suitable for large-scale production to ensure stable protein expression and high yield.
Clinical sample production: Protein production is carried out in a GMP compliant facility to ensure product quality and batch to batch consistency.
Quality control system: Strict quality control measures are implemented, including inspection of raw materials, intermediate products and final products to prevent microbial contamination, such as bacteria and mycoplasma.
Drug grade release testing: Following drug grade release testing standards, a comprehensive quality assessment of recombinant protein products is performed to ensure their suitability for preclinical and clinical study phases.
Supplier management: relatively fixed the main raw materials, packaging materials suppliers, and adhere to the quality assessment of suppliers to ensure the quality stability of raw materials.
Expert consultation and certification: For products that are transitioning from research Use (RUO) to GMP grade, it is important to seek expert help to make the transition smoothly and obtain official GMP certification.
Continuous improvement and monitoring: Throughout the production process, the process needs to be continuously optimized and monitored to ensure product quality and production efficiency.
Documentation and traceability: Detailed records of each step of the operation and results, in order to facilitate product traceability and quality control.

Applications
- Cell therapy: GMP-grade proteins such as cytokines and growth factors play a key role in stem cell therapy, affecting the proliferation, differentiation and other properties of stem cells to help them better adapt to therapeutic needs.
- Fusion protein drugs: In the production of fusion protein drugs, GMP grade protein is an important raw material, and its quality standard has a decisive impact on the quality of the final drug product.
- Preclinical studies: GMP-grade proteins can be used for cell culture and differentiation, as well as for demanding cell culture medium preparation, which is essential for preclinical studies. Due to its low endotoxin, no mycoplasma and virus contamination, it can achieve large-scale production, reduce costs and ensure product stability.
- Tumor immunotherapy: In tumor immunotherapy, GMP-grade proteins such as cytokines are not only used to prepare and culture CAR-T cells, but also to enhance anti-tumor effects as a third signal.
- Regenerative Medicine Research and production: In the field of regenerative medicine, GMP-grade proteins help promote cell proliferation and differentiation to repair damaged tissues.
Case Study
Case Study 1: Active GMP Recombinant Human CD19 protein
Although it has been shown that the production of functional chimeric antigen receptor T cells is feasible in patients with B-cell malignancies, it is currently unclear whether sufficient amounts of functional autologous CAR T cells can be generated from patients with autoimmune diseases. Intrinsic T-cell abnormalities and T-cell-targeted immune suppression in patients with autoimmunity may hamper the retrieval of sufficient T cells and their transduction and expansion into CAR T cells. This material was used as source for manufacturing anti-CD19 CAR T-cell products (CAR) in clinical scale. Cells were transduced with a lentiviral anti-CD19 CAR vector and expanded under good manufacturing practice (GMP) conditions using a closed, semi-automatic system. Functionality of these CAR T cells derived from autoimmune patient cells was tested in vitro. Six SLE patients were analyzed. Leukapheresis could be successfully performed in all patients yielding sufficient T-cell numbers for clinical scale CAR T-cell production. In addition, CAR T cells showed high expansion rates and viability, leading to CAR T cells in sufficient doses and quality for clinical use. CAR T cells from all patients showed specific cytotoxicity against CD19+ cell lines in vitro. GMP grade generation of CD19 CAR T-cell products suitable for clinical use is feasible in patients with autoimmune disease.
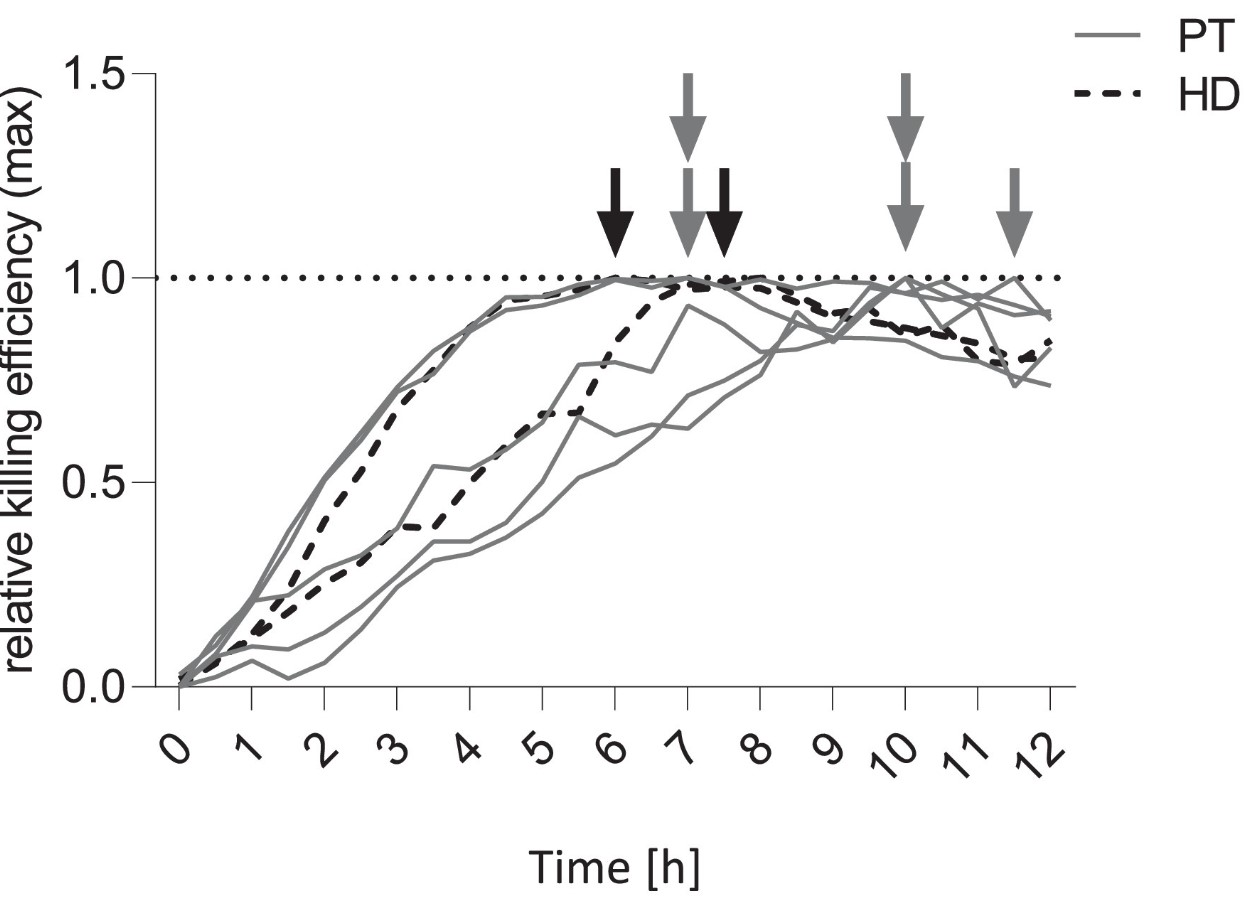
(S Kretschmann, 2023)
Fig1. Kinetics of CAR-mediated killing: Arrows indicate the time of maximal cytotoxic activity of HD- and patient-derived CAR T cells against a CD19+ target cell line.
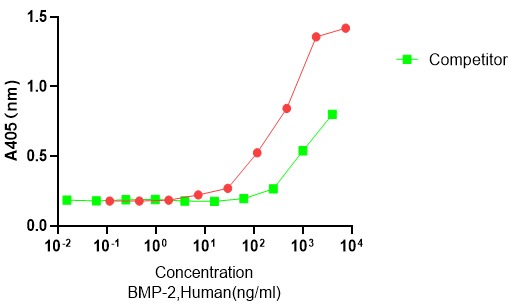
Case Study 2: GMP Recombinant Human BMP2
The aim of the present study was to assess the recombinant bonemorphogenetic protein 2 (RHBMP-2) with higher substantively and solubility for use in calcium phosphate scaffolds for better release in differentiation of mesenchymal stem cells to osteoblast cells. Using bioinformatics tools, two mutations (p. L10D and p. S12E) were chosen and applied in BMP2 CDS sequence to increase interaction with calcium derived composite. The new recombinant mutated sequence (BMP2mut ) was synthesized and then subcloned to expression vector pBV220. Experimental data regarded functional protein expression in E. coli. Since no modification was made in the active sites of proteins namely β-sheets and α-helixes, not only was there any change in the specific activity occurred in the specific activity of the enzyme in comparison to its commercial counterpart, but also mesenchymal osteogenesis occurred more efficient on biphasic CaP scaffold model. As hypothesized, use of negatively charged amino acids such as aspartate and glutamate in protein loops increased the interactions of BMP2-Ca2+ and resulted in its slower and more sustained released from CaP scaffolds compare to commercial RHBMP2.
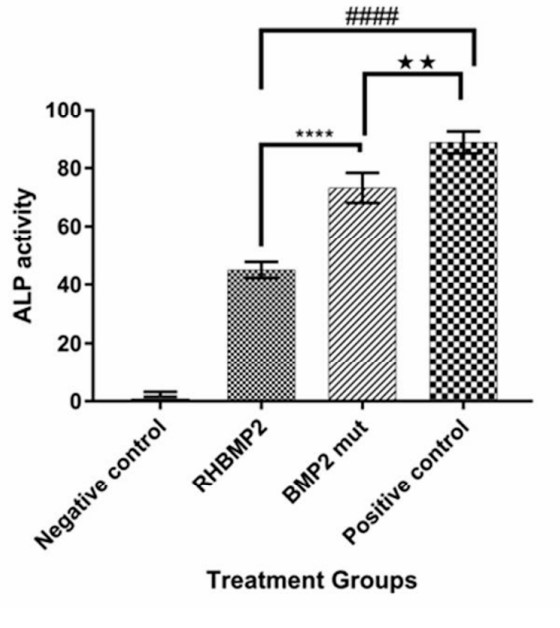
(Mohammad Bayat, 2017)
Fig2. Calcium content assay after MSc differentiation. Negative control is MSc without BMP2 or inductive medium culturing. RHBMP2 and BMP2mut group means MSc that cultured in present of them.
Case Study 3: Active GMP Recombinant Human CD34 protein
Based on a synergistic consortium, the cord blood (CB) bank Düsseldorf was responsible for the selection of HLA-homozygous (HLA-h) donors, contacting/re-consenting the mothers, Good Manufacturing Practice (GMP)-grade CD34+ enrichment, followed by short-term expansion of CD34+ cells and qualification of the resulting CD34+ population as advanced therapy medicinal product (ATMP)-starting material. Among 20 639 licensed Düsseldorf cord blood units (CBUs), 139 potential HLA-h donors were identified with the most frequent 10 German haplotypes. 100% of the donors were contacted, and for 47·5%, consent was obtained. HLA-A, -B, -C, -DR, -DQ and -DP were determined by sequencing. Thawing/washing of the CBUs was performed in the presence of Volulyte/HSA with Sepax® , CD34+ selection by automated CliniMACS® -system (Miltenyi), expansion with qualified GMP-grade cytokines and media in the GMP facility. Here, we specify minimal criteria (≥5 x 105 viable CD34+ -count, ≥80% CD34+ -purity and ≥70% viability) and confirm that n = 10 CB units (max storage time 16 years) could be qualified for an ATMP starting material. The mean fold change expansion of isolated CD34+ cells at Day 3/4 (d3/4) was 3·38 ± 3·02 with a mean purity of 86·90 ± 10·38% and a high viability of 96·07 ± 4·72%.
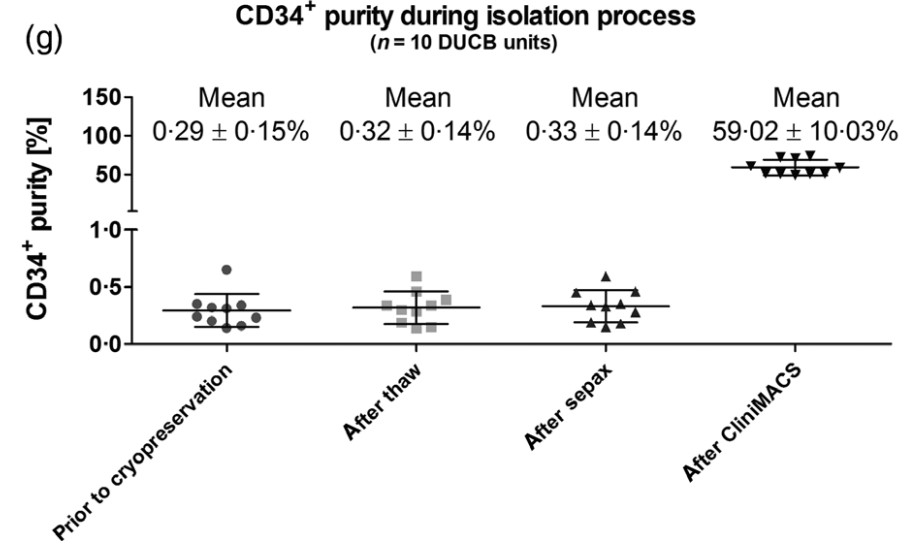
(Stefanie Liedtke, 2021)
Fig3. Qualitative analysis during CD34+ isolation. Viable CD34+-purity.
Case Study 4: GMP Recombinant Human CCL21
The previous studies have demonstrated that transduction of human dendritic cells (DC) with adenovirus encoding secondary lymphoid chemokine, CCL21, led to secretion of biologically active CCL21 without altering DC phenotype or viability. In addition, intratumoral injections of CCL21-transduced DC into established murine lung tumors resulted in complete regression and protective anti-tumor immunity. These results have provided the rationale to generate a clinical grade adenoviral vector encoding CCL-21 for ex vivo transduction of human DC in order to assess intratumoral administration in late stage human lung cancer. In the current study, human monocyte-derived DC were differentiated by exposure to GM-CSF and IL-4 from cryopreserved mononuclear cells obtained from healthy volunteers. Transduction with clinical grade adenoviral vector encoding CCL21 (1167 viral particles per cell) resulted in secretion of CCL21 protein. CCL21 protein production from transduced DC was detected in supernatants (24-72 hours, 3.5-6.7 ng/4-5 x 10(6) cells). DC transduced with the clinical grade adenoviral vector were > 88% viable (n = 16), conserved their phenotype and maintained integral biological activities including dextran uptake, production of immunostimulatory cytokines/chemokines and antigen presentation. Furthermore, supernatant from CCL21-DC induced the chemotaxis of T2 cells in vitro.
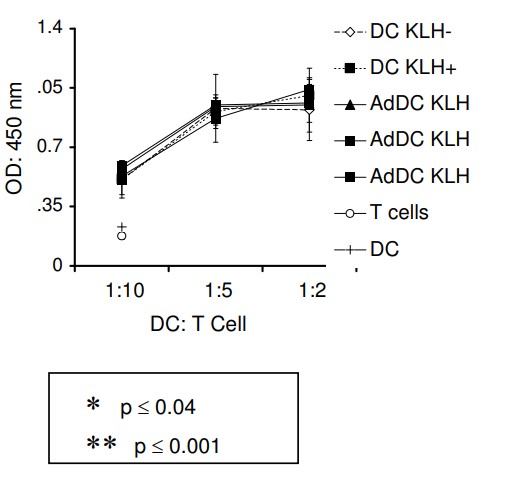
(Felicita Baratelli, 2008)
Fig4. Allogeneic MLR: Briefly, culture medium mock-transduced DC (DC) and CCL21-DC (AdDC) were analyzed for their ability to induce allogeneic T cell proliferation.
Advantages
- Strict quality control: We have high-quality production facilities and equipment, as well as a strict quality management system to ensure the quality and consistency of our products.
- Professional team: The production of GMP grade proteins requires specialized knowledge and technology, and our R&D team covers experts in biology, chemistry, pharmacy and other fields.
- Certification and Compliance: Companies that we can provide GMP grade protein services are often required to pass relevant certifications to demonstrate that products and services meet international standards and regulatory requirements.
About Us
To ensure batch-to-batch consistency and high product quality, Creative BioMart pays extraordinary attention to details at all levels of the manufacturing process. Our GMP-grade proteins are completely free of human- and animal-derived components and manufactured and tested under a certified ISO 9001:2008 quality system, with extensive documentation for every step of the manufacturing process. Highest product standards are ensured by rigorous quality control (QC) tests. They meet all of the requirements with relevant GMP guidelines.
We do rigorous quality control tests:
- N-terminal sequence analysis
- Molecular weight determination by MS and SDS-PAGE
- HPLC analysis
- Residual Host Cell DNA Content
- Residual Host Cell Protein Content
- Endotoxin
- Mycoplasma
- Biological activity
- Other tests depending on your needs

Our GMP-grade proteins are generated from the same clone and expression system as the research-grade proteins. This enables us to smoothly switch between GMP-grade and research-grade proteins. Please feel free to ask for custom production of GMP-grade proteins. We offer both small-scale and large-scale GMP production, formulation, and fill/finish. Wide range of manufacturing scales from 10L to 500L can meet all your needs from clinical research to commercial production.
References
- Bayat M.; et al. Protein engineering of recombinant human bone morphogenetic protein 2 with higher interaction with Ca phosphate based scaffold used for osteogenesis. J Biomed Mater Res A. 2017;105(10):2799-2805.