Wnt Signal Pathway
🧪 LRP5-29428TH
Source: Wheat Germ
Species: Human
Tag: Non
Conjugation:
Protein Length: 90 amino acids

🧪 WNT3-5160M
Source:
Species: Mouse
Tag: Non
Conjugation:
Protein Length:
🧪 CDC42-11008H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-186a.a.
🧪 DAAM1-11811H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 715-1068a.a.
🧪 GSK3A-13561H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: C-term-78a.a.
🧪 JNK-211H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-427aa
🧪 PFN1-1658H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-140aa
🧪 WNT11-3737H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-354aa
🧪 WNT3-3738H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-355aa
🧪 WNT4-3740H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-351 aa

🧪 WNT8B-3741H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 197-267aa

🧪 Lrp5-608M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: Met1-Ser1383
🧪 Wnt5a-2055M
Source: E.coli
Species: Mouse
Tag: GST&His
Conjugation:
Protein Length: Ile62~Lys380
🧪 Rock1-15M
Source: E.coli
Species: Mouse
Tag: His
Conjugation:
Protein Length: Leu1094~Gly1323
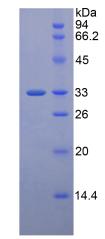
🧪 LRP5-7870H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: Asp769~Leu1016
Background of Wnt Signal Pathway
What is Wnt Signal Pathway?
The Wnt signal pathway is a complex network of proteins involved in a variety of crucial biological processes, including cell-to-cell communication, embryonic development, tissue homeostasis, and cell differentiation. The pathway is named after its discovery as a regulator of wingless (Wg) gene expression in Drosophila and the integration of the Wnt gene in mice. The Wnt signaling pathway can be divided into several types, but the most well-known are the canonical (or Wnt/β-catenin) pathway and the non-canonical pathways, which include the Wnt/Ca2+, Wnt/PCP, and Wnt/Rho pathways.
The Canonical Wnt/β-catenin Signal Pathway
The hallmark of the canonical Wnt signal pathway is the accumulation and translocation of adhesion-related protein β-catenin within the nucleus. Without Wnt signaling, β-catenin in the cytoplasm is degraded by β-catenin destruction complex, which includes Axin, adenomatosis protein APC, protein phosphatase 2A (PP2A), glycogen synthase kinase 3 (GSK3), and casein kinase 1α (CK1α). Among them, CK1α and GSK3 are responsible for β-catenin phosphorylation, making it ubiquitinated and subsequent proteolytic destruction by the proteosomal machinery.
The complex of Wnt with its receptors Fz and LRP5/6 triggers a series of activities that target the destruction complex of β-catenin. Binding of Wnt to the Fz / LRP5/6 complex induces membrane translocation of the key signaling negative regulator Axin, which binds to the conserved sequence of the LRP5/6 cytosolic tail. Axin binding to LRP5/6 during its membrane translocation is catalyzed by CK1γ or GSK3-mediated phosphorylation of LRP5/6. Importantly, CK1 and GSK3 play different roles at two levels of the canonical Wnt signal pathway. At the LRP5/6 level, they act as a positive regulator, and at the β-catenin level, they act as a negative regulator. Binding of Axin to LRP5/6 abolishes the negative activity of Axin on Wnt signaling, which in some way leads to the activation of Dsh. Once Dsh protein is activated, it inhibits the activity of GSK3 enzyme and activates a series of complex signals to stop the degradation of β-catenin and induce its stability and accumulation in the cytoplasm. Stabilized β-catenin then enters the nucleus while the mechanism is not yet clear. The β-catenin has no nuclear localization sequence (NLS) and does not require importin proteins or Ran-mediated nuclear import into the nucleus. One hypothesis currently proposed is that β-catenin is "piggybacked" to the nucleus by other factors, possibly including Axin, which also appears to undergo nuclear shuttling. There is also no nuclear export sequence (NES) on β-catenin. Current research has identified two mechanisms for this process. One is related to Ran binding protein 3 (RanBP3) and the NES-bearing APC protein; the other is directly involved in nuclear pore complexes independent of Ran. In the nucleus, β-catenin acts as a transcriptional coactivator to exert its effects on gene transcription. A large number of nuclear β-catenin binding partners have been identified, of which the most representative is the DNA-binding transcription factor LEF/TCF.
The Non-Canonical Wnt Signal Pathway
-
The Planar Cell Polarity (PCP) Signal Pathway
The PCP signal pathway emerged from the genetic study of Drosophila in which mutations in Wnt signaling proteins Fz and Dsh randomize the orientation of epithelial structures, including the stratum corneum and the sensory mane. Fz co-receptors of non-canonical PCP signaling have not been specifically identified and demonstrated, and candidate factors include NRH1, Ryk, PTK7 and ROR2. The signal is then passed on to the Dsh, resulting in the activation of Dsh. The PDZ and DEP domains of Dsh were used to activate two parallel pathways respectively that activate the small GTPases Rho and Rac. In order to activate the Rho signaling branch, Wnt signaling induces the formation of the Dsh-Daam1 complex and activates Daam1. Dishevelled associated activator of morphogenesis 1 (Daam1) is a Formin homology protein that, in combination with Dsh and RhoA, suggests a positive feedback loop signal. Activated Rho induces activation of Rho-associated kinase (ROCK) and myosin, resulting in modification of the actin cytoskeleton and cytoskeletal rearrangement. The second branch requires the DEP domain of Dsh to activate Rac. This activation is independent of Daam1 and the activated Rac in turn activates JNK. JNK downstream factors of non-canonical signal pathways are still unknown. Although both Rho and Rac are involved in transcriptional regulation, it remains unclear what genes are regulated by these two GTPases in the non-canonical Wnt signal pathway.
-
The Wnt/Ca2+ Signal Pathway
The second branch of the non-canonical Wnt signal pathway is the Wnt/Ca2+ signal pathway. Although sharing many components of the PCP signal pathway, this pathway also has a clear distinction. This pathway can further regulate the classic Wnt/β-catenin signal pathway as well as the PCP signal pathway. The Wnt/Ca2+ pathway revealed that Wnt and Fz receptors stimulate intracellular Ca2+ release from the endoplasmic reticulum (ER), which is dependent on the G protein. Wnt5a, Wnt11 and Fz2 promote intracellular Ca2+ release without affecting the stability of β-catenin. Ca2+ release, which is caused by over-expression of Wnt5a and Fz2 in zebrafish embryos, can be inhibited by pertussis toxin and the alpha subunit of transducin, which also inhibit G protein signaling. These studies indicate that Wnt/Fz signaling induces intracellular Ca2+ release via the G protein. Ca2+ release and intracellular accumulation activate various Ca2+ sensitive proteins, including PKC and calmodulin-dependent kinase II (CamKII). Studies have shown that CamKII activates the transcription factor NFAT to regulate the fate of abdominal cells in the Xenopus embryos. CamKII also activates TGFβ kinase TAK1 and Nemo-like kinase (NLK) to inhibit β-catenin/TCF signaling. Ca2+ can also activate PKC, which regulates the process of tissue separation during gastrulation by activating the small GTPase CDC42. Taken together, these studies suggest that the Wnt/Ca2+ signal pathway plays an important regulatory role in both classical Wnt signaling and non-classical PCP signaling.
3. The Wnt/Rho Signal Pathway
The Wnt/Rho signal pathway is an important branch of the non-classical Wnt signaling pathway, which mainly regulates cell migration, polarity and histomorphogenesis. Activation of this pathway begins with binding of the Wnt ligand to the Frizzled receptor, which in turn affects the activity of the Rho family of GTPase enzymes, including RhoA, Rac1, and Cdc42. These GTPases are involved in regulating the dynamic recombination of the cytoskeleton, controlling cell morphology, adhesion and migration. The Wnt/Rho signaling pathway plays a key role in many biological processes such as embryonic development, tissue repair, tumor invasion and neurodevelopment. Abnormal Wnt/Rho signaling activation is associated with pathological states such as cancer metastasis, cardiovascular disease, and neurodegenerative diseases, making it a potential target for the treatment of these diseases.
Core Components of Wnt Signal Pathway
Wnt Ligands: Wnt ligands are a group of secreted glycoproteins that activate the Wnt signaling pathway by binding to cell surface receptors. There are 19 members of the Wnt family in vertebrates, and each member has its specific biological function and mechanism of action. Wnt ligands act on receptors on adjacent or the same cell by autocrine or paracrine means.
Frizzled (Fz) receptor: Frizzled is a membrane bound receptor in the Wnt signaling pathway and belongs to the G protein-coupled receptor family. They transmit signals by binding to Wnt ligands. Activation of the Frizzled receptor is a key step in the Wnt signaling pathway, which participates in a variety of Wnt signaling pathways, both classical and non-classical.
Beta – catenin: β-catenin is a core molecule in the Wnt/β-catenin signaling pathway. In the absence of a Wnt signal, beta-catenin typically binds to a degradation complex in the cytoplasm and is then phosphorylated and targeted for degradation. When Wnt signaling is activated, β-catenin accumulates and transfers to the nucleus, binds to TCF/LEF family transcription factors, and activates the expression of Wnt target genes.
Disheveled (Dsh): Disheveled protein plays a pivotal role in the Wnt signaling pathway and participates in signal transduction. In the classical Wnt signaling pathway, Dsh activation helps to inhibit the phosphorylation and degradation of β-catenin, thereby allowing β-catenin to accumulate and activate target genes. In the non-classical Wnt signaling pathway, Dsh is involved in a variety of different signaling events, including cell polarity, migration, and proliferation.
GSK-3β (Glycogen Synthase Kinase 3β): GSK-3β is a versatile serine/threonine kinase that plays a key regulatory role in the Wnt/β-catenin signaling pathway. When the Wnt signal is not activated, GSK-3β is involved in the phosphorylation of β-catenin, labeling it for ubiquitination and proteasome degradation. Activation of Wnt signaling stabilizes the β-catenin protein by inhibiting the activity of GSK-3β.
Wnt Signal Pathway Related Diseases
Wnt signal pathway involves in many complex cellular physiological activities and biochemical reactions, the dysfunction can lead to a variety of diseases. Abnormal activation of Wnt signaling or loss of intercellular adhesion function can induce tumorigenesis. The key role of β-catenin is out of control, which leads to colon cancer and rectal cancer. The Wnt signal pathway has both oncogenes and tumor suppressor genes that play important roles in the development of many human malignancies. Wnt signaling also regulates the proliferation and differentiation of hematopoietic stem cells and mesenchymal stem cells. The role of Wnt protein in embryonic development is obvious, affecting the formation of central nervous system, limbs, and heart, which can lead to a variety of birth defects and cardiovascular disease.
Case Study
Case Study 1: Recombinant Human WNT3A (WNT3A-1700H)
Human pluripotent stem cells (hPSCs) can provide a platform to model bone organogenesis and disease. To reflect the developmental process of the human skeleton, hPSC differentiation methods should include osteogenic progenitors (OPs) arising from three distinct embryonic lineages: the paraxial mesoderm, lateral plate mesoderm, and neural crest. To generate lineage-specific OPs from human embryonic stem cells and human induced pluripotent stem cells, we employed stepwise differentiation of paraxial mesoderm-like cells, lateral plate mesoderm-like cells, and neural crest-like cells toward their respective OP subpopulation. We also report, for the first time, high FGF1 levels in neural crest-derived OPs—a notable finding given the critical role of fibroblast growth factors (FGFs) in osteogenesis and mineral homeostasis. For osteogenic commitment, PM, LP, and NC cells were kept in the osteogenic basal medium including Wnt3a for 6 days. Our results indicate that FGF1 influences RUNX2 levels, with concomitant changes in ERK1/2 signaling.

Fig1. Flow cytometric analysis showing marker expression of PM cells derived from hiPSCs. (Fahad Kidwai, 2020)
Case Study 2: Recombinant Mouse Fzd1 protein (Fzd1-2292M)
Postinfarct cardiac hypertrophy is an independent risk factor for heart failure and sudden death. Regression of cardiac hypertrophy has emerged as a promising strategy in the treatment of myocardial infarction (MI). Here the researchers hypothesized that frizzled1 (FZD1), a receptor of the canonical Wnt signaling pathway, is a novel mediator of ischemia-associated cardiac hypertrophy. MI was induced in mice by left anterior descending (LAD) coronary occlusion. One week after MI, the expression of FZD1 was found to be notably increased in the left ventricles (LVs) of the MI-mice compared to shams. Mouse recombinant FZD1 protein (RFP) was subcutaneously injected in the mice to provoke autoimmunization response. Anti-FZD1 antibody titer was significantly increased in the plasma of RFP-treated mice. RFP significantly mitigated the MI-induced cardiac hypertrophy and improved cardiac function in the MI mouse hearts. Moreover, increased heart and LV weights, myocardial size and the expression of β-myosin heavy chain in the MI-mice were also found to be attenuated by RFP. FZD1 was found to be significantly up-regulated in hypoxia-treated neonatal rat cardiomyocytes (NRCMs). Silencing FZD1 by siRNA transfection notably repressed the hypoxia-induced myocardial hypertrophy in NRCMs.

Fig2. Quantitative results of the protein expression of FZD1 in NRCMs. (Jingjing Fan, 2017)
Case Study 3: Recombinant Mouse Lrp5 Protein (Lrp5-608M)
The Wnt signaling pathway is intricately connected with bone mass regulation in humans and rodent models. The researchers designed an antibody-based platform that generates potent and selective Wnt mimetics. Using this platform, they engineer bi-specific Wnt mimetics that target Frizzled and low-density lipoprotein receptor-related proteins and evaluate their effects on bone accrual in murine models. These synthetic Wnt agonists induce rapid and robust bone building effects, and correct bone mass deficiency and bone defects in various disease models, including osteoporosis, aging, and long bone fracture. Furthermore, when these Wnt agonists are combined with antiresorptive bisphosphonates or anti-sclerostin antibody therapies, additional bone accrual/maintenance effects are observed compared to monotherapy, which could benefit individuals with severe and/or acute bone-building deficiencies.

Fig3. Fzd1–10, along with Lrp5, and Lrp6 mRNA expression in mesenchymal stem cells (MSC). (Lucas B Murray, 2012)
Related Products
The Wnt signal pathway is an evolutionarily conserved signal pathway that regulates cell migration, cell polarity, and neural and organ formation during embryonic development. The major signaling downstream of the Frizzled (Fz) receptor have been identified, including the canonical Wnt/β-catenin signal pathway and the non-canonical β-catenin-independent signal pathway. Creative BioMart can provide a list of core protein products of the Wnt pathway to help you with your researches. Please feel free to contact us if you’re interested.

