Growth Factors

🧪 BMP2-01H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 283-396 aa
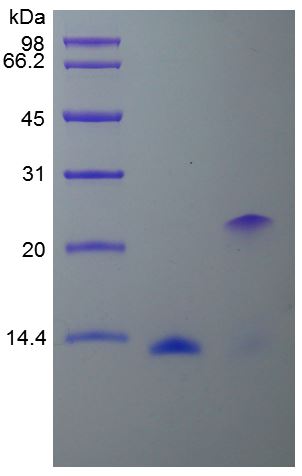

🧪 CSF3-02H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
🧪 CSF2-03H
Source: E. coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 18-144 aa
$199.00
$398
/ 100μg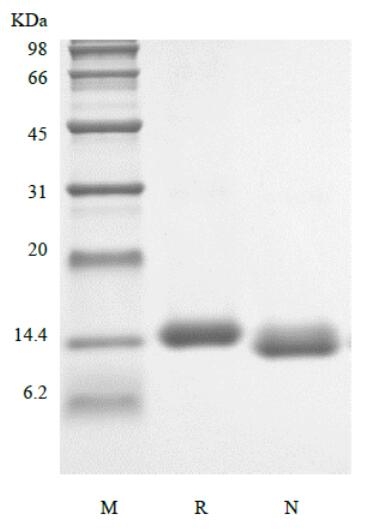

🧪 EGF-04H
Source: E.coli
Species: Human
Tag:
Conjugation:
Protein Length:
$149.00
$298
/ 1mg

🧪 FGF2-11H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
$199.00
$398
/ 100μg$699.00
$1,398
/ 1mg

🧪 IGF1-06H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 70
🧪 IGF-08H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
$119.00
$238
/ 100 µg
🧪 VEGFA-17H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 166
$99.00
$198
/ 10μg
🧪 Vegfa-18M
Source: P.pastoris
Species: Mouse
Tag: Non
Conjugation:
Protein Length: Ala27-Arg190

🧪 EPO-01H
Source: Mammalian Cells
Species: Human
Tag: Non
Conjugation:
Protein Length:
🧪 IL1A-01H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 159
$74.50
$149
/ 10μg$399.00
$798
/ 100μg$849.00
$1,698
/ 500μg

🧪 IL1B-02H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 153

🧪 IL4-10H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 129 amino acids


🧪 IL6-12H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 183
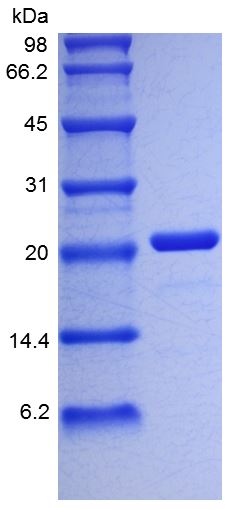

🧪 NGF-05H
Source: CHO
Species: Human
Tag: Non
Conjugation:
Protein Length: Ser122-Arg239
Growth Factors—Product Overview
Growth factors are essential signaling molecules that regulate a wide range of cellular processes, including proliferation, differentiation, migration and survival. They act through specific receptors to activate signaling pathways critical for development, tissue repair, and immune responses. Dysregulation of growth factor signaling is implicated in many diseases, including cancer and fibrosis. Creative BioMart offers a comprehensive range of high-quality recombinant growth factors and related products to support research in cell biology, regenerative medicine, oncology and therapeutic development.
Jump to Section
Background
Growth factors are a class of organic substances that are essential for the regulation of normal growth and metabolism of microorganisms, but cannot be synthesized de novo from simple carbon and nitrogen sources. They are polypeptides that regulate cell growth and other functions by binding specifically and with high affinity to cell membrane receptors. They are present in platelets and various adult and embryonic tissues, as well as in most cultured cells, with some degree of specificity for different cell types. Typically, the growth of cultured cells requires the coordinated action of multiple growth factors in a defined sequence. Tumor cells have the property of growing autonomously, independent of growth factors.
Classification of Growth Factors
Growth factors are typically classified based on structure, receptors they bind to, or biological functions. We offer a comprehensive selection covering all major growth factor families:
-
Epidermal Growth Factor (EGF)
Stimulates epithelial cell growth, proliferation, and wound healing.
-
Fibroblast Growth Factor (FGF)

Regulates cell proliferation, differentiation, migration, and angiogenesis.
-
Vascular Endothelial Growth Factor (VEGF)
Promotes the formation of new blood vessels (angiogenesis).
-
Platelet-derived Growth Factor (PDGF)

Stimulates cell growth and division, especially in blood vessels and connective tissues.
-
Placental Growth Factor (PGF)

Modulates angiogenesis, particularly in pregnancy and pathological conditions.
-
Transforming Growth Factor-beta (TGF-β)
Cell growth inhibition and immune regulation.
-
Growth Differentiation Factor (GDF)

Promotes development, repair, and cell differentiation, especially in tissues.
-
Glial Cell-derived Neurotrophic Factor (GDNF)
Supports survival and growth of neurons, especially motor and dopaminergic.
-
Bone Morphogenetic Proteins (BMPs)
Induces bone and cartilage development; involved in embryogenesis.
-
Insulin-like Growth Factor (IGF) Family
Regulates growth, development, metabolism; mimics insulin effects broadly.
-
Hepatocyte Growth Factor (HGF)
Stimulates liver regeneration and influences cell motility and morphogenesis.
-
Angiopoietin (ANG)

Modulates blood vessel maturation and stability.
-
Macrophage-stimulating protein (MSP)

Promotes macrophage activation and cell motility.
-
Hepatoma-derived growth factor (HDGF)
Involved in cell growth, angiogenesis, and tumor development.
-
Thrombopoietin (TPO)

Regulates the production of platelets from megakaryocytes.
-
Adrenomedullin (AM)

Functions in vasodilation and angiogenesis.
Signaling Pathways
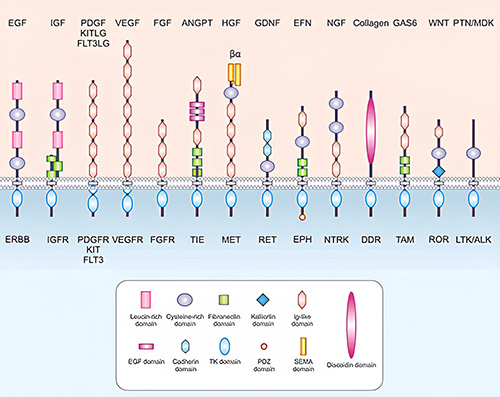
Growth factors exert their biological effects by binding to specific cell surface receptors, primarily receptor tyrosine kinases (RTKs). Upon ligand binding, these receptors undergo dimerization and autophosphorylation, triggering a cascade of intracellular signaling pathways such as the MAPK/ERK, PI3K/AKT, and JAK/STAT pathways. These signaling networks regulate critical cellular processes, including proliferation, differentiation, migration, and survival. Precise control of these pathways is essential for normal development, tissue homeostasis, and wound healing. Aberrant activation of growth factor signaling is implicated in several diseases, particularly cancer, making these pathways important targets for therapeutic intervention and biomedical research.
Applications
Stem Cell Research and Regenerative Medicine
Growth factors play a critical role in maintaining the pluripotency of stem cells, driving their differentiation into specialized cell types, and promoting tissue regeneration. They are widely used to engineer tissues such as skin, cartilage, and even entire nerves in the laboratory.
Wound Healing and Tissue Repair
Many growth factors, such as EGF and FGF, accelerate the healing process by stimulating cell proliferation, migration, and extracellular matrix production. They are used in the clinical treatment of burns, ulcers, and surgical recovery.
Cancer Research and Oncology
Because growth factors are often hijacked by tumors to promote uncontrolled growth and angiogenesis, they are intensively studied to better understand cancer progression and to develop targeted therapies or inhibitors.
Cell Culture and Expansion
In vitro cell culture relies heavily on growth factors to maintain healthy, proliferative cells. For example, EGF is routinely added to epithelial cell cultures, while FGF supports fibroblast and stem cell growth.
Pharmaceutical Development and Drug Screening
Growth factors are vital tools for creating disease models and testing new drug candidates. They help mimic physiological conditions, making screening assays more predictive of real-world outcomes.
Cardiovascular and Neural Regeneration
Specific growth factors such as VEGF and NGF (nerve growth factor) are being studied for their ability to stimulate blood vessel formation and nerve repair, with exciting potential in the treatment of heart disease and neurodegenerative disorders.
Cosmetic and Aesthetic Medicine
Because of their regenerative powers, growth factors are also a hot topic in skin rejuvenation treatments, hair regrowth therapies and anti-aging products.
Product Features
-
Broad and Comprehensive Portfolio: We offer an extensive range of growth factor proteins, covering key families such as EGF, FGF, VEGF, BMPs, and beyond, to support researchers in regenerative medicine, oncology, and cell biology.
-
High Purity and Bioactivity: All growth factors are produced using advanced expression systems and stringent purification processes to ensure exceptional purity and robust biological activity for reliable, reproducible results.
-
Multiple Species and Expression Options: Available in human, mouse, and other species variants, with flexible expression platforms including E. coli, mammalian cells, and insect cells to match diverse experimental needs.
-
Customization Services: From bulk production to specific isoforms or activity-optimized variants, our custom solutions help you find the perfect fit for your research or product development pipeline.
-
Strict Quality Control: Every batch undergoes rigorous testing for identity, purity (SDS-PAGE, HPLC), endotoxin levels, and bioactivity assays—because consistency isn’t just a goal, it's a promise.
-
Expert Technical Support: Our scientific team is available for consultation, protocol optimization, and troubleshooting-so you're never left in the dark when it matters most.
Case Study
Case 1: Improved EPO Doping Analysis
Reihlen et al., 2024. Introduction of a PEGylated EPO conjugate as internal standard for EPO analysis in doping controls. Drug Testing and Analysis, doi:10.1002/dta.3211.
Immunopurification of doping control samples is a mandatory necessity in erythropoietin (EPO) analysis during a confirmation procedure; moreover, it has become common practice to also immunopurified samples for the initial testing procedure. The presented data demonstrate that a 12-kDa PEG residue attached to human EPO represents a particularly useful construct to serve as ISTD for erythropoietin-receptor agonist (ERA) analysis. The conjugate is applicable to both urine and blood testing using the commonly employed purification techniques, supporting and improving result interpretations especially concerning specimens where the natural abundance of human EPO is low.
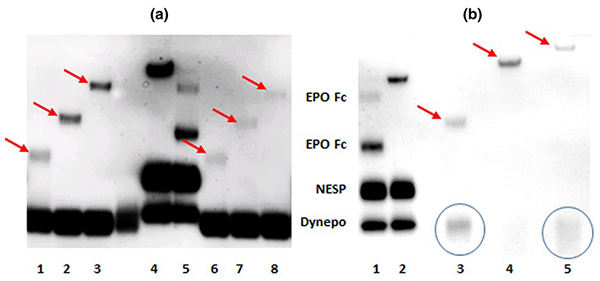
Figure 1. (a) Immunoblot after sodium N-lauroylsarcosinate (“sarcosyl”) polyacrylamide gel electrophoresis (SAR-PAGE) of urine samples spiked with different Recombinant erythropoietin-polyethylene glycol (rEPO-PEG) conjugates. rEPO was conjugated with PEGs of varying molecular mass via aldehyde (Ald) or N-hydroxysuccinimide ester (NHS) linkage: 5-kDa Ald (1), 10-kDa Ald (2), 20-kDa Ald (3), ESA-Mix CERA-NESP-Dynepo (4), ESA-Mix EPO Fc-NESP-Dynepo (5), 5-kDa NHS (6), 10-kDa NHS (7), and 20-kDa NHS (8). (b) Immunoblot after SAR-PAGE of different directly applied rEPO-PEG conjugates. ESA-Mix EPO Fc-NESP-Dynepo (1), ESA-Mix CERA-NESP-Dynepo (2), 12-kDa NHS (3), 40-kDa Ald (4), and 60-kDa Ald (5).
Case 2: Dual Growth Factor Delivery
Patel et al., 2015.Dual delivery of EPO and BMP2 from a novel modular poly-ɛ-caprolactone construct to increase the bone formation in prefabricated bone flaps. Tissue Engineering, doi:10.1089/ten.tec.2014.0643.
Poly-ɛ-caprolactone (PCL) is a biocompatible polymer that has mechanical properties suitable for bone tissue engineering; however, it must be integrated with biologics to stimulate bone formation. Bone morphogenetic protein-2 (BMP2) delivered from PCL produces bone when implanted subcutaneously, and erythropoietin (EPO) works synergistically with BMP2. In this study, EPO and BMP2 are adsorbed separately on two 3D-printed PCL scaffold modules that are assembled for codelivery on a single scaffold structure. BMP2 and EPO scaffolds had more ingrowth (1.4%±0.6%) in the outer module when compared with BMP2 (0.8%±0.3%) at 4 weeks. Dual delivery produced more dense cellular marrow, while BMP2 had more fatty marrow. Dual EPO and BMP2 delivery is a potential method to regenerate bone faster for prefabricated flaps.
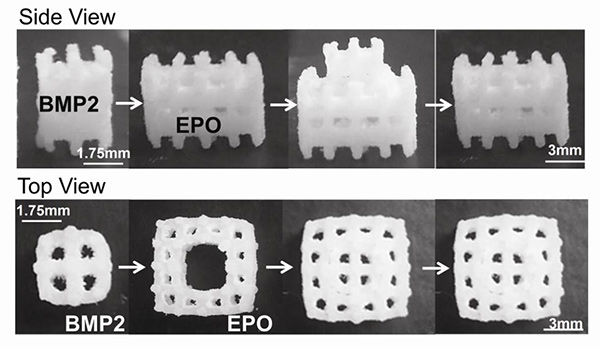
Figure 2. Modular scaffold assembly. BMP2 was adsorbed onto the inner scaffold module and EPO was adsorbed onto the outer scaffold module. The two scaffolds were then assembled. BMP2, bone morphogenetic protein-2; EPO, erythropoietin.
Case 3: Induced Dopamine Neuron Generation
Playne et al., 2018. Generation of dopamine neuronal-like cells from induced neural precursors derived from adult human cells by non-viral expression of lineage factors. J Stem Cells Regen Med, doi: 10.46582/jsrm.1401005.
Reprogramming technology holds great promise for the study and treatment of Parkinson's disease (PD) as patient-specific ventral midbrain dopamine (vmDA) neurons can be generated. This should facilitate the investigation of early changes occurring during PD pathogenesis, permitting the identification of new drug targets and providing a platform for drug screening.
Previous reports have indicated that induced neural precursors (iNPs) derived from adult human fibroblasts by lineage factor-mediated direct reprogramming can give rise to dopamine neurons expressing tyrosine hydroxylase (TH+). Using normal adult human fibroblasts, the present study aimed to extend these findings and determine the capacity of iNPs for generating vmDA neurons, with the aim of utilising this technology for the future study of PD.
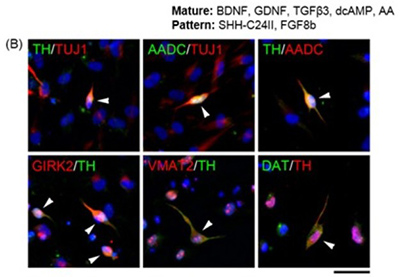
Figure 3. Expression of dopaminergic markers in cultures differentiated with Patterning and Maturation media. Arrowheads indicate cells with positive staining.
FAQs
-
Q: What are the differences between growth factors and cytokines?
A: Growth factors and cytokines are both small, secreted proteins that regulate cell behavior, but they have different biological roles and contexts.
- Growth factors primarily promote cell proliferation, differentiation and survival. They are essential for development, wound healing and tissue regeneration. Examples include EGF, FGF, VEGF, and PDGF.
- Cytokines, on the other hand, are more involved in immune responses, inflammation, and cell signaling between immune cells. They include interleukins (ILs), interferons (IFNs), and tumor necrosis factors (TNFs).
Structurally and mechanistically, there's overlap - some proteins (such as GM-CSF) can even be classified as both. However, growth factors are typically associated with tissue development and maintenance, while cytokines are strongly associated with immune system regulation.
-
Q: What expression systems are used for your recombinant growth factor proteins?
A: We use a range of optimized expression systems including E. coli, mammalian cells (HEK293, CHO), and insect cells, depending on the structural and functional needs of each growth factor. This ensures high purity, proper folding, and optimal biological activity for your research applications.
-
Q: Are your growth factors bioactive? How is bioactivity tested?
A: Yes, all of our growth factors are verified for biological activity using cell-based functional assays. Each lot undergoes rigorous validation, such as proliferation or phosphorylation assays, to ensure consistent and reproducible performance in your experiments.
-
Q: How pure are your recombinant growth factors?
A: Our recombinant proteins typically exceed 95% purity, as confirmed by SDS-PAGE and HPLC analysis. Endotoxin levels are strictly controlled, making them suitable for sensitive in vitro and in vivo studies. -
Q:Do you offer different isoforms or species variants of growth factors?
A: Yes! We offer a variety of isoforms and species-specific variants, including human, mouse, rat, and others. If you need a specific form that isn't listed, our custom manufacturing services can provide a solution tailored to your specifications.
-
Q: Can you provide bulk quantities or GMP grade growth factors?
A: Absolutely. We offer scalable production from research grade to preclinical and GMP grade materials to support your needs from early research through clinical development. Bulk discounts and flexible packaging options are also available. -
Q: How should I store and handle recombinant growth factor proteins?
A: We recommend storing lyophilized proteins at -20°C to -80°C. After reconstitution, the protein should be aliquoted to avoid repeated freeze-thaw cycles and stored at -80°C for optimal stability. Detailed handling instructions are provided with each product.
-
Q: What if I can't find the product I need in the list?
A: If you can't find the protein you need in the product list, you can search for the protein using the search bar at the top of the site. If you still can't find the product, or if you have a special requirement for the protein, feel free to contact us directly.
-
Q: Can you help me choose the right growth factor for my experiment?
A: Yes! Our experienced technical support team is available to help you select the most appropriate growth factor based on your experimental system, cell type, or research objective. Please feel free to contact us for a consultation.
Related Products
- Transforming Growth Factor beta (TGF-β) Superfamily
- BMP Subfamily
- Interleukin
- Colony-Stimulating Factor (CSF)
- GDNF Family Ligands
- GDNF Family Receptors
- Fibroblast Growth Factor (FGF)
- Platelet-Derived Growth Factor (PDGF) Family
- Growth Differentiation Factor (GDF)
- Epidermal Growth Factor (EGF)
- Insulin-like Growth Factor (IGF)
- Vascular Endothelial Growth Factors (VEGFs)
- Cytokines

