mTOR Signaling Pathway
🧪 KRAS-2569H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-185 aa

🧪 EIF4E-2775H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-217 aa

🧪 AKT3-131H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-479 a.a.
🧪 TNFRSF1B-178H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met1-Asp257
🧪 RAF1-1123H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 50-132 a.a.
🧪 RHOA-1145H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
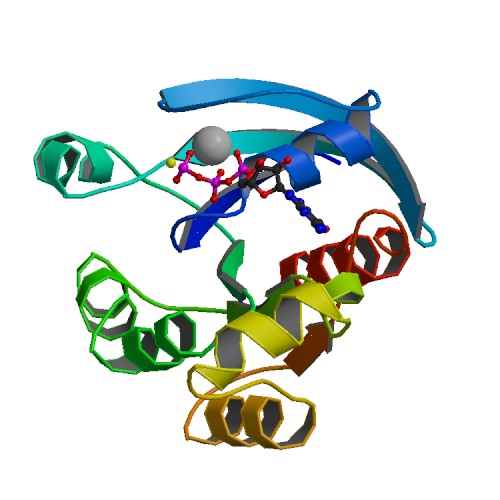
🧪 EIF4EBP1-1397H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-118 a.a.

🧪 FZD5-713H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-167 a.a.
🧪 GSK3B-616H
Source: Insect Cells
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Thr433
🧪 RAC1-2213H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: Met1-Cys189

🧪 Fzd1-2292M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: 1-248 a.a.
🧪 TNFRSF1A-3189H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Thr211
🧪 AKT1-786H
Source: Insect Cells
Species: Human
Tag: His
Conjugation:
Protein Length: 1-480 aa
🧪 Tnfrsf1a-3306M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: 1-212 a.a.
🧪 Tnfrsf1b-3274M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: 1-258 a.a.
Background of mTOR Signaling Pathway
What is mTOR Signaling Pathway?
The mTOR Signaling Pathway is a critical intracellular signaling network that plays a pivotal role in regulating various cellular processes, including cell growth, metabolism, proliferation, and survival. At the heart of this pathway is the mammalian target of rapamycin (mTOR), a serine/threonine kinase that integrates a wide range of signals from the extracellular environment and intracellular conditions to modulate cellular functions.
The mTOR pathway consists of two structurally and functionally distinct complexes: mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2). These complexes are composed of different subunits that confer unique sensitivities and specific cellular roles.
Critical Components and Functions
mTORC1
mTORC1 is sensitive to the macrolide antibiotic rapamycin (also known as sirolimus) and
is primarily involved in regulating cell growth and metabolism. It is composed of mTOR itself, Raptor, mLST8,
PRAS40, and DEPTOR. When activated, mTORC1 stimulates anabolic processes such as protein synthesis and lipid
biosynthesis, while inhibiting catabolic processes like autophagy. The activation of mTORC1 is influenced by various
signals, including growth factors, nutrient availability (especially amino acids), energy status, and stress
conditions. Key upstream regulators of mTORC1 include the TSC complex and the Rheb GTPase, which modulate the
complex's activity based on nutrient and energy levels. TSC is a heterotrimeric complex comprised of TSC1, TSC2, and
TBC1D7, and functions as a GTPase activating protein (GAP) for the small GTPase Rheb that directly binds and
activates mTORC1.
mTORC2
In contrast to mTORC1, mTORC2 is not sensitive to rapamycin and is involved in
regulating cell survival, proliferation, and cytoskeletal organization. It is composed of mTOR, Rictor, mLST8,
Deptor, mSIN1, and Protor1/2. mTORC2 plays a crucial role in modulating the activity of the Akt kinase, which is
involved in promoting cell survival and growth. Additionally, mTORC2 has been implicated in the regulation of the
PKC family of kinases, which are important for cytoskeletal dynamics. mTORC2 primarily functions as an effector of
insulin/PI3K signaling. Like most PI3K regulated proteins, the mTORC2 subunit mSin1 contains a
phosphoinositide-binding PH domain that is critical for the insulin-dependent regulation of mTORC2 activity.

Fig1. The components of mTORC1 and mTORC2, and the most well known substrates of the two complexes. (Jose J Limon, 2012)
Physiological Roles of mTOR Signaling Pathway
The mTOR signaling pathway plays a multitude of physiological roles in the body, acting as a central regulator of cell growth, metabolism, proliferation, and survival. Here are some of the key physiological roles of the mTOR signaling pathway:
Cell Growth and Proliferation: mTORC1, one of the two main complexes of the mTOR pathway, is
sensitive to nutrients and growth factors, and it promotes cell growth and proliferation by stimulating protein
synthesis and lipid biosynthesis. It does this by activating ribosomal protein S6 kinase (S6K) and inhibiting
eukaryotic translation initiation factor 4E-binding protein (4EBP), which are key regulators of translation
initiation.
Metabolic Regulation: The mTOR pathway integrates signals from nutrients, energy
status, and growth factors to regulate cellular metabolism. mTORC1 is particularly involved in the regulation of
glucose metabolism, where it can modulate glycolysis and glucose uptake in response to glucose levels. It also plays
a role in lipid metabolism by controlling the expression of genes involved in fatty acid
synthesis.
Autophagy: mTORC1 acts as a negative regulator of autophagy, a process by which
cells degrade and recycle their own components. Under nutrient-deficient conditions, mTORC1 is inhibited, leading to
the activation of autophagy, which helps to maintain cellular homeostasis and energy balance.
Cell
Survival and Apoptosis: mTORC1 and mTORC2 both play roles in promoting cell survival. mTORC1 does this
by inhibiting pro-apoptotic proteins and activating anti-apoptotic proteins. mTORC2, on the other hand, has been
shown to activate the pro-survival kinase Akt, which is a key mediator of cell survival signals.
Immune
Function: The mTOR pathway is also involved in the regulation of the immune system. mTORC1 is crucial
for the differentiation and function of various immune cells, including T cells and macrophages. It can modulate the
balance between pro-inflammatory and anti-inflammatory responses, which is essential for maintaining immune
homeostasis.
Aging and Longevity: Studies have shown that inhibition of mTORC1 can extend
lifespan in various organisms, including yeast, worms, and flies. In mammals, reduced mTORC1 signaling has been
associated with increased lifespan in certain genetic models, suggesting that mTORC1 may be a potential target for
promoting health and longevity in humans.
Response to Stress: mTORC1 is sensitive to various
forms of cellular stress, including oxidative stress and DNA damage. It can modulate the cellular response to these
stresses by regulating cell cycle progression and apoptosis, thereby influencing the overall stress tolerance of the
cell.
Cardiovascular Health: mTORC1 and mTORC2 have been implicated in the regulation of
cardiovascular health. mTORC1 inhibition has been shown to protect against cardiac hypertrophy and ischemic damage,
while mTORC2 has been linked to the regulation of heart function and metabolism.

Fig2. Pharmacological targeting of the mTOR signaling cascade in human malignancies. (Vivek Panwar, 2023)
mTOR Signaling Pathway Related Diseases
The mTOR signaling pathway is
implicated in a wide range of diseases due to its central role in regulating cell growth, metabolism, proliferation,
and survival. Here are some of the key diseases associated with the mTOR signaling pathway:
Cancer: Aberrant activation of the mTOR pathway is frequently observed in various types of cancer.
The mTORC1 complex, in particular, has been linked to cell proliferation and survival, making it a target for cancer
therapy. Inhibition of mTORC1 can suppress tumor growth and angiogenesis, while mTORC2 is also being investigated
for its role in cancer cell survival and resistance to chemotherapy.
Cardiovascular Diseases:
mTOR signaling plays a role in the development of cardiovascular diseases, including atherosclerosis, cardiac
hypertrophy, and heart failure. Both mTORC1 and mTORC2 have been shown to regulate cardiovascular processes such as
lipid metabolism, inflammation, and the response to ischemia.
Neurodegenerative Diseases: The
mTOR pathway is involved in the pathogenesis of neurodegenerative diseases like Alzheimer's, Parkinson's, and
Huntington's disease. mTORC1 activation has been associated with the dysregulation of autophagy, which can lead to
the accumulation of toxic proteins and neuronal damage.
Autoimmune Diseases: mTOR signaling is
crucial for the regulation of immune cell function, and dysregulation of mTORC1 and mTORC2 has been implicated in
autoimmune diseases such as multiple sclerosis and rheumatoid arthritis. mTOR inhibitors have shown promise in the
treatment of these conditions by modulating immune responses and reducing inflammation.
Metabolic
Disorders: The mTOR pathway is central to the regulation of metabolism, and its dysregulation can lead
to metabolic disorders like obesity and type 2 diabetes. mTORC1, in particular, is involved in the regulation of
glucose and lipid metabolism, and its inhibition has been shown to improve insulin sensitivity and reduce metabolic
syndrome features.
Aging and Age-Related Diseases: mTOR signaling is known to influence the
aging process and the development of age-related diseases. Inhibition of mTORC1 has been shown to extend lifespan in
model organisms, and it is thought to have potential in delaying the onset of age-related diseases by promoting
cellular health and reducing inflammation.
Inflammatory Diseases: The mTOR pathway is involved
in the regulation of inflammatory responses, and its activation can contribute to the development of inflammatory
diseases. mTOR inhibitors have been studied for their potential to reduce inflammation and improve outcomes in
conditions such as inflammatory bowel disease.
Case Study
Case Study 1: Recombinant Human EIF4E (EIF4E-2775H)
The researchers have previously reported 18S and 25S ribosomal RNA molecules in Candida albicans resistant to processive 5' → 3' exonuclease, appearing as cells approached stationary growth phase. Initial analysis pointed to extra phosphate(s) at their 5'- end raising the possibility that they were newly transcribed. Here we report on additional experiments exploring this possibility and try to establish which of the RNA polymerases may be transcribing them. Oligo-ligation and primer extension again showed the presence of extra phosphate at the 5'-end of the reported processing sites for both 18S and 25S ribosomal RNA components. Inhibition of Pol I with BMH-21 increased the presence of the molecules. Quantitation showed that resistant 18S and 25S molecules are primarily produced in the nucleus. Both the cap specific antibody and eIF4E cap-binding protein, increased fold enrichment upon quantitative amplification. Antibodies specific for the RNA Polymerase II c-terminal domain and TFIIB initiator factor showed the presence of Pol II on DNA sequences for both 18S and 25S molecules in chromatin precipitation and qPCR assays. Rapamycin inhibition of TOR complex also resulted in an increase of resistant 18S and 25S molecules.

Fig1. RT-qPCR quantification of 18S, 25S and ITS-1 molecules precipitated from total RNA by eIF4E cap binding protein (CBP). (Ilaria Moscetti, 2018)
Case Study 2: Recombinant Human TP53 protein (TP53-185H)
p53 is found to be functionally inactivated by mutations in many human tumors. Accordingly, wild type p53 and its oncogenic mutants represent valuable cancer biomarkers for diagnostic and prognostic purposes. The researchers developed a highly sensitive biosensor, based on Surface Enhanced Raman Spectroscopy, for detection of wild type p53 and of p53R175H, which is one of the most frequent tumor-associated mutants of p53. By following the enhanced signal of a specific Raman marker, intrinsic to the nanoparticle-antibody bioconjugation, they were able to push the antigen detection level down to the attomolar range in buffer and to the femtomolar range in spiked human serum. The method demonstrated a high reproducibility and a remarkable selectivity in discriminating between wild type p53 and p53R175H mutant, in both buffer and serum. A calibration plot was built and validated by ELISA for a reliable quantification of p53. The approach could be easily extended to ultrasensitive detection of other markers of general interest, with feasible implementations into multiplex assays, functioning as lab-on-chip devices for several applications.

Fig2. SERS spectra of the samples obtained upon incubating the anti-p53wt probe on substrates functionalized with p53wt at different concentrations. (Anna Rita Bizzarri, 2018)
Case Study 3: Recombinant Human BCL2
Mechanistic target of rapamycin mTOR complex 1 (mTORC1) plays a key role in the integration of various environmental signals to regulate cell growth and metabolism. mTORC1 is recruited to the lysosome where it is activated by its interaction with GTP-bound Rheb GTPase. However, the regulatory mechanism of Rheb activity remains largely unknown. Here, this study shows that ubiquitination governs the nucleotide-bound status of Rheb. Lysosome-anchored E3 ligase RNF152 catalyzes Rheb ubiquitination and promotes its binding to the TSC complex. EGF enhances the deubiquitination of Rheb through AKT-dependent USP4 phosphorylation, leading to the release of Rheb from the TSC complex. Functionally, ubiquitination of Rheb is linked to mTORC1-mediated signaling and consequently regulates tumor growth. Thus, a mechanistic model is proposed whereby Rheb-mediated mTORC1 activation is dictated by a dynamic opposing act between Rheb ubiquitination and deubiquitination that are catalyzed by RNF152 and USP4 respectively.

Fig3. RNF152 enhanced Rheb ubiquitination in vitro. (Lu Deng, 2019)
Related Products
mTOR signaling pathway is a key signal transduction network in the cell, which interacts and coordinates with many other signaling pathways to regulate cell growth, metabolism, proliferation and survival. We have compiled key proteins involved in the mTOR signaling pathway into one handbook for your reference. Meanwhile, Creative BioMart can provide a list of related protein products to support your ongoing research. Please feel free to contact us if you’re interested.


