Bmp2
-
Official Full Name
bone morphogenetic protein 2 -
Overview
The protein encoded by this gene belongs to the transforming growth factor-beta (TGFB) superfamily. The encoded protein acts as a disulfide-linked homodimer and induces bone and cartilage formation. [provided by RefSeq, Jul 2008] -
Synonyms
BMP2;bone morphogenetic protein 2;BDA2;BMP2A;BMP-2A;bone morphogenetic protein 2A
Recombinant Proteins
- Human
- Human/Mouse/Rat/Rhesus/Canine
- Mouse
- Rat
- Cattle
- Dog
- Pig
- Rabbit
- Chicken
- E.coli
- HEK293
- CHO
- Nicotiana Tobacum
- Human
- Mammalian Cells
- Wheat Germ
- Human Cells
- Yeast
- Non
- GST
- Fc
- His
- S
- T7
- Avi
- SUMO
Background
What is bmp2 protein?
The BMP2 protein, or Bone Morphogenetic Protein 2, is a transformative member of the growth factor-beta superfamily extensively implicated in innumerable developmental processes. The inherent property of BMP2 to stimulate bone and cartilage growth, as a regenerative and non-mitogenic extracellular protein, has led to the utilization of BMP2 as a therapeutic agent for bone healing and repair.
BMP2 was first discovered during the 1960s through studies focused on osteoinductive substances. The exact timeframe of BMP2's discovery is somewhat fuzzy, owing to the multitude of intrinsic components involved in bone development. Historically, the discovery of BMP2 was a significant stride in the study of developmental biology, as it highlighted the existence of secreted proteins that could direct cellular fate.
The BMP2 gene is situated on the short arm of chromosome 20 at locus 20p12. The gene encompasses seven exons, with transcription resulting in a precursor protein that is subsequently cleaved to generate the mature protein. The BMP2 protein structure comprises a monomer that is part of a larger homodimer or heterodimer complex. This larger complex is stabilized by a disulfide bond and demonstrates a distinct 'cystine knot' like configuration.
What is the function of bmp2 protein?
BMP2 is instrumental in inducing bone and cartilage formation. It regulates growth, differentiation, chemotaxis, and apoptosis in a myriad of cell types, including mesenchymal cells, epithelial cells, hematopoietic cells, neuronal cells, etc. Essentially, BMP2 acts as a vital regulator during embryogenesis and postnatal development, molding various organs, including teeth, limbs, heart, and the nervous system. In adults, its role further extends to maintaining homeostasis of tissues such as bone and cartilage.
Bmp2 related signaling pathway
BMP2 signaling is performed chiefly via canonical SMAD-dependent and non-canonical SMAD-independent pathways. The SMAD-dependent pathway is the primary signal transduction pathway for TGF-β superfamily members, including BMP2. Here, transduced signals from cell surface to nucleus regulate gene transcription. Conversely, the SMAD-independent pathways include MAPK pathway, PI3K/Akt pathway, and PKC pathway, primarily involved in the regulation of cytoskeletal dynamics and cell mobility.
BMP2 Related Diseases
- Fibrodysplasia Ossificans Progressiva (FOP): Also known as "Stone Man Syndrome," this is a rare disease in which the body's soft tissues progressively turn into bone. BMP2, along with other proteins, play a significant role in the abnormal bone growth.
- Heterotopic Ossification (HO): This condition also involves abnormal bone growth in areas like muscles and tendons. Increased levels of BMP2 can kick start the process of ossification, leading to this condition.
- Atherosclerosis: BMP2 plays a role in the formation of bone-like tissue in the arteries, a critical aspect of atherosclerosis.
- Congenital Heart Defects: Children born with heart defects may have mutations in the BMP2 gene. It plays an important role in heart development, and disruptions can cause defects.
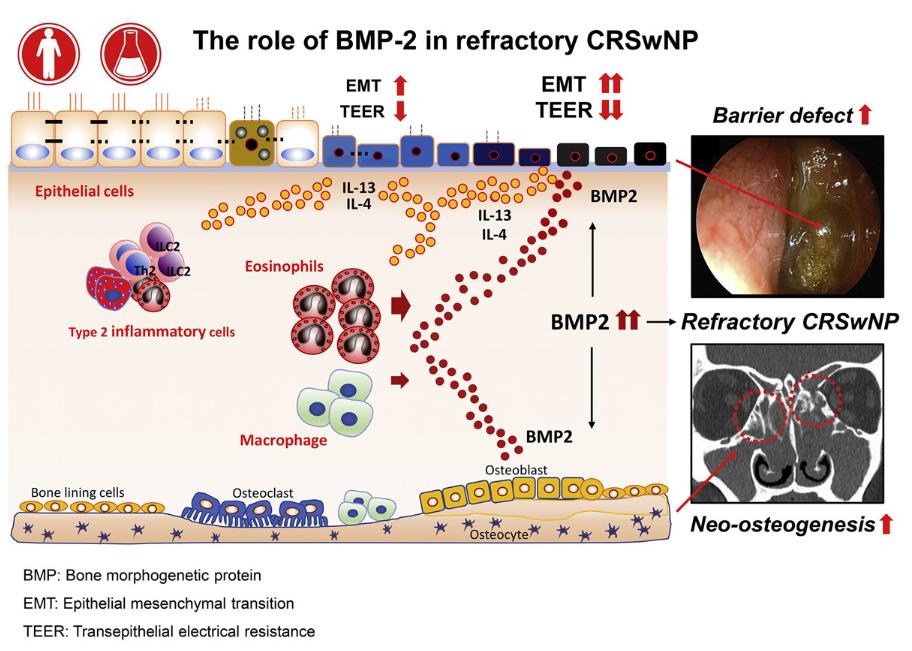
Bone morphogenetic protein-2 as a novel biomarker for refractory chronic rhinosinusitis with nasal polyps (Kim, J. Y., 2021)
Biomedical Application of BMP2 Protein
- Bone Regeneration: BMP2 is used as a clinical tool to stimulate bone regeneration. It is used in spinal fusion, fractures, and also in dental implants where bone growth is required.
- Cartilage Repair: BMP2 can stimulate chondrocytes, the cells that produce cartilage, making it potentially beneficial for cartilage repair.
- Cancer Treatment: Due to its role in cell growth and death, BMP2 can potentially be used as a novel therapeutic target or diagnostic biomarker for various cancers.
- Wound Healing: Studies suggest BMP2 can enhance wound healing and tissue repair, potentially shortening recovery times after surgery or injury.
Case Study
Case study 1: Janki J Patel, 2015
One strategy to reconstruct large bone defects is to prefabricate a vascularized flap by implanting a biomaterial scaffold with associated biologics into the latissimus dorsi and then transplanting this construct to the defect site after a maturation period. This strategy requires the ability to quickly (<1 h within an operating room) and efficiently bind biologics to scaffolds. It also requires the ability to localize biologic delivery.
In this study, the efficacy of binding bone morphogenetic protein-2 (BMP2) to poly-ɛ-caprolactone (PCL) was investigated using adsorption and conjugation as a function of time. Adsorbed 65 μg/mL BMP2 solution resulted in the greatest regenerated bone volume (15.0±3.0 mm3), elastic modulus (20.1±3.0 MPa), and %bone ingrowth in the scaffold interior (17.2%±5.4%) when compared with conjugation.
Thus this article indicates that adsorption may be optimal for the clinical application of prefabricating bone flaps due to BMP2 binding in a short exposure time, retained BMP2 bioactivity, and bone growth adhering to scaffold geometry and into pores with healthy marrow development.
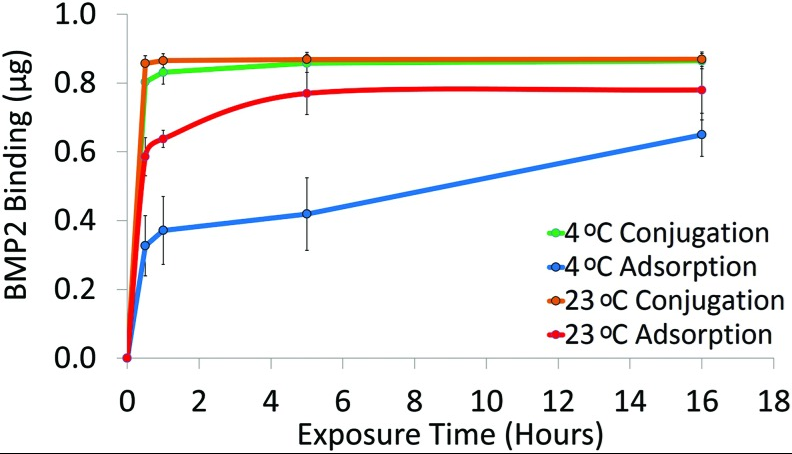
Fig1. BMP2 binding to PCL discs via adsorption or conjugation. PCL discs were exposed to 1.4 μg/mL BMP2solution for 0.5, 1, 5, or 16 h at 23°C or 4°C. BMP2 was quantified with an ELISA (n=3). BMP2, bonemorphogenetic protein-2; ELISA, enzyme-linked immunoabsorbant assay.
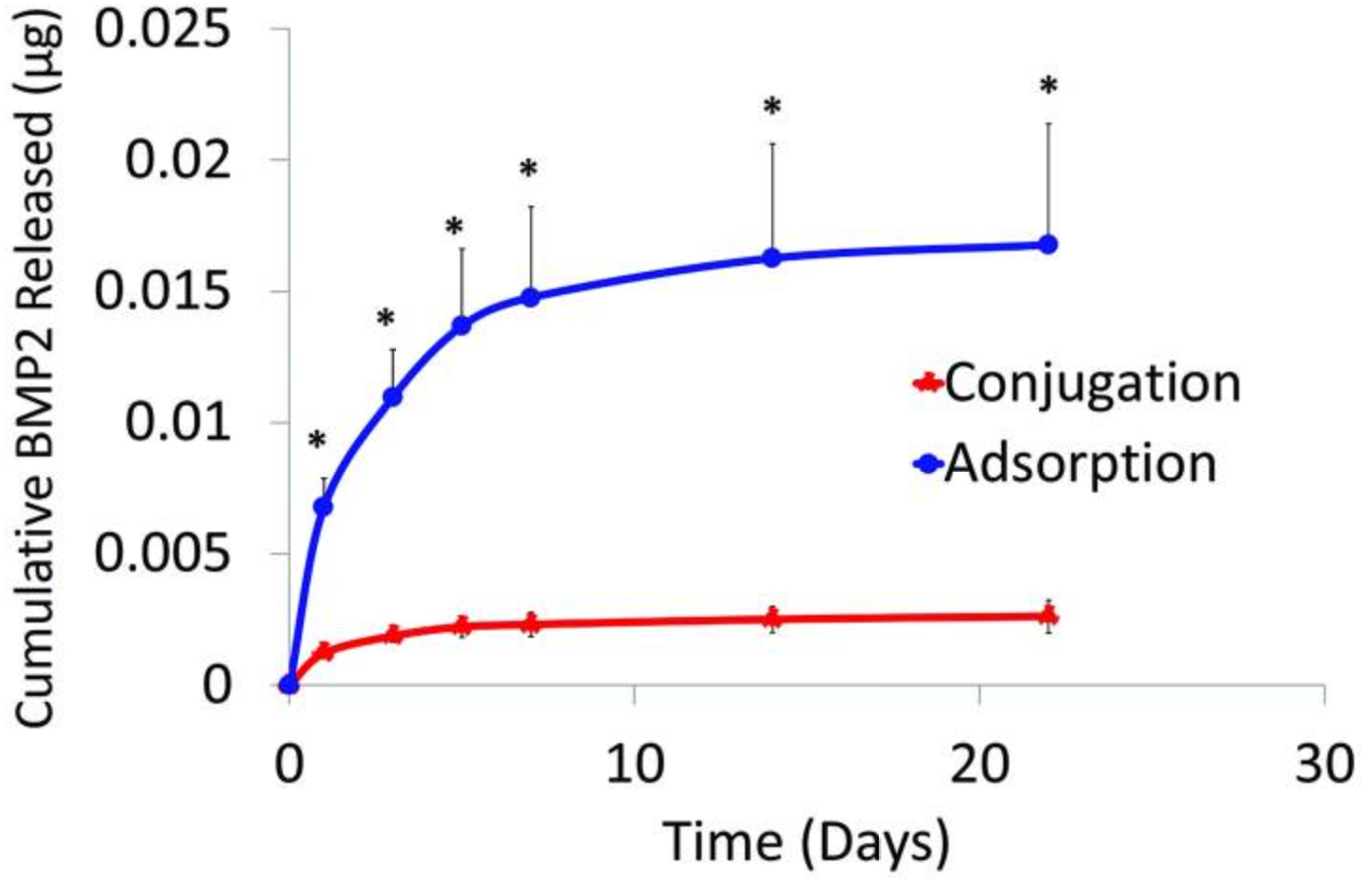
Fig2. Conjugated and adsorbed BMP2 released from PCL. Cumulative release of BMP2 from PCL scaffolds into DPBS whenexposed to 20 μg/mL BMP2 for 1 h at 23°C. Release environment conditions were sterile,37°C, 5% CO2, and 95% humidity. *p<0.05
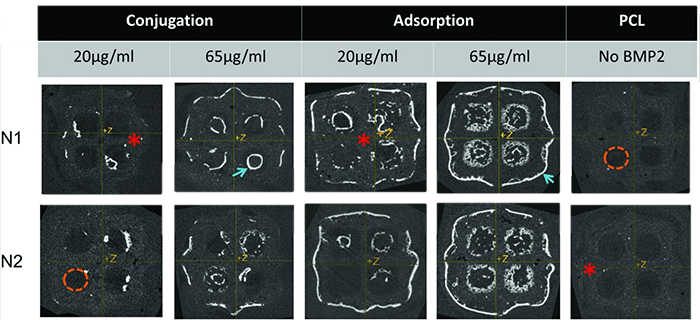
Fig3. Conjugation produced bone that closely followed PCL surface geometry. Adsorption produced bone growth into the pores in addition to following surface geometry. Two representative samples from each group are shown. Bright white areas indicate bone formation (blue arrow), and gray areas are scaffold (red*). Dark areas (orange dashed lines) indicate pores.
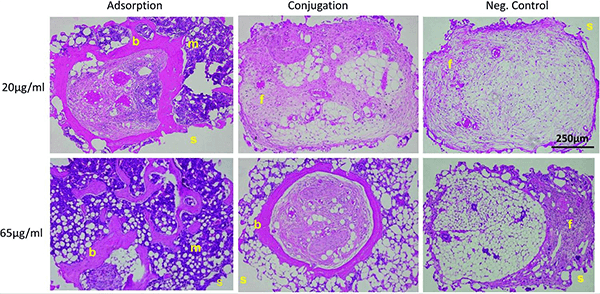
Fig4. Hematoxylin and eosin images of PCL/BMP2 scaffold pores. Both of the adsorbed groups (20 and 65?μg/mL) showed blood, bone, and fatty marrow growth into the scaffold pore space. Bright-field images of scaffold pores taken at 10× magnification. b, bone; f, fibrous tissue; m, marrow; s, scaffold. Negative controls consisted mainly of fibrous tissue.
Case Study 2: Ali H Hassan, 2016
The aim of the present study was to develop and examine a new non-invasive injectable graft for the repair of alveolar bone clefts using recombinant human bone morphogenetic protein-2 (rhBMP-2) encapsulated within injectable liposomal in situ gel (LIG).
Different liposomal formulations loaded with rhBMP-2 were prepared, and the effects of the preparation methods and lipid content on the efficiency of rhBMP-2 encapsulation within the liposomes were studied. Critical size alveolar defects were surgically created in the maxillae of 30 New Zealand rabbits and treated with different injectable formulae, including rhBMP-2 liposomes and in situ gel (rhBMP-2-LIG).
The results indicated that the prepared rhBMP-2-LIG prolonged the release and residence time of BMP-2 within rabbits for more than 7 days. BMP-2-LIG is a promising delivery device for the repair of alveolar bone defects associated with cleft deformities.
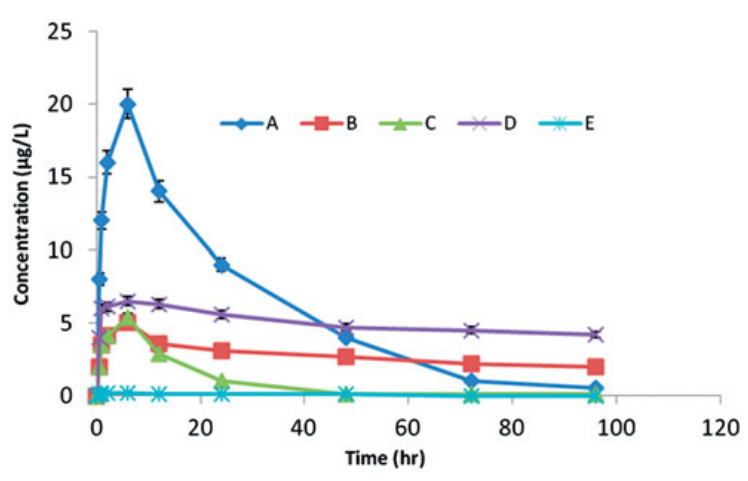
Fig1. Mean plasma levels of BMP-2 after the local injection of 5 mg/kg of BMP-2 isotonic saline into defects inrabbits. These experimental variables were optimum for the release of rhBMP-2, reflecting the availability of thiscompound at the defect. (A), BMP-2 in unilamellar liposomal suspension (B), BMP-2 dispersed in the in situ gel(C),rhBMP-2-LIG-3 formula (D) and negative control (E).
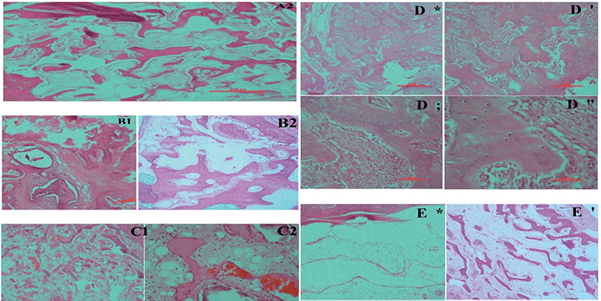
Fig2. The newly formed bone was surrounded with fibro-vascular tissues and this defect might be fully repaired withtime. Photomicrograph of a bone defect in Group A, Group B (slides stained with H&E, at 4×(B1) and 40×(B2)), Group C(slides stainedwith H&E, at 4×(C1) and 40×(C2)), Group D (slides stained with H&E, at 4×(D*,’) and40×(D;,”)) and Group E (slides stained with H&E, at 4×(E*) and 10×(E’).
Quality Guarantee
High Purity
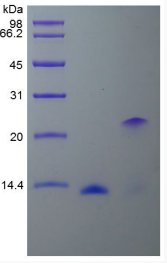
Fig1. SDS-PAGE (BMP2-01H) (PROTOCOL for western blot)
High Bioactivity & Detection Sensitivity
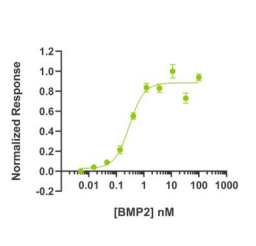
Fig2. BMP2 activity Bioactivity is determined using a BMP2-responsive firefly luciferase reporter in stably transfected HEK293T cells. Cells are treated with a serial dilution of BMP2 for 6 hours. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity. EC50 = 7.81 ng/mL (0.3 nM).
Involved Pathway
Bmp2 involved in several pathways and played different roles in them. We selected most pathways Bmp2 participated on our site, such as Cytokine-cytokine receptor interaction,Hedgehog signaling pathway,TGF-beta signaling pathway, which may be useful for your reference. Also, other proteins which involved in the same pathway with Bmp2 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| TGF-beta signaling pathway | TGIF2,SMAD7,PPP2CA,E2F5,PITX2,FST,ZFYVE9,BMP7A,ACVR1BA,SPAW |
| Hippo signaling pathway | PPP2R2B,SOX2,WNT9A,FZD7,SCRIB,FZD5,BBC3,PPP2R2D,TEAD1,PARD6A |
| Cytokine-cytokine receptor interaction | NGFRB,PDGFAB,TGFB2,GM13304,TNFRSF14,IFNA5,EDA2R,BMPR2,IL15RA,CCL25 |
| Hedgehog signaling pathway | WNT3,FBXW11B,GSK3AA,DYRK1A,PRKACAA,CSNK1DB,SKI,WNT9A,GLI1,SAP18 |
| Basal cell carcinoma | WNT8B,Shh,FZD3,AXIN1,DVL3,FZD2,TCF7L2,WNT10B,WNT9B,WNT7B |
| Signaling pathways regulating pluripotency of stem cells | HAND1,PIK3CG,ACVR2A,AXIN2,HOXB1,FZD8,ONECUT1,GRB2,REST,IGF1 |
| Pathways in cancer | BIRC7,MSH3,ITGA3,BCL2L1,PGF,MMP1A,RALGDS,GNB2,GNG3,IL6 |
Protein Function
Bmp2 has several biochemical functions, for example, BMP receptor binding,SMAD binding,co-receptor binding. Some of the functions are cooperated with other proteins, some of the functions could acted by Bmp2 itself. We selected most functions Bmp2 had, and list some proteins which have the same functions with Bmp2. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| cytokine activity | GDF15,FAM3D,GREM1A,GREM2,Ifna15,SPAW,CCL38A.5,CCL38.1,IL3,IL12BA |
| BMP receptor binding | BMP7B,BMP5,BMP8B,BMP8A,BMP2B,BMP3,PYCARD,BMP4,BMP7A,BMP6 |
| co-receptor binding | HFE,TFR2,DKK1,BMP4,NEO1 |
| growth factor activity | MDKA,INHBA,PRL2C2,BMP3,BMP8A,VHL,MANF,PDGFAA,Il2,CECR1A |
| protein binding | KIAA1274,PHOSPHO2-KLHL23,BET1,OLFM1,DDR2,DEDD2,PHF7,GLYAT,VPS8,CUL4B |
| SMAD binding | TCF12,USP15,SKOR1,SKIB,MEF2A,HMGA2,SMURF2,YY1,TGFBR2,TGFBRAP1 |
| receptor binding | RASA1,DVL3,CCL15,APLN,CRH,FGF18A,KCNA5,CD72,PVRL3B,WIPI1 |
| retinol dehydrogenase activity | HSD17B6,SDR16C5,ADH7,RDH12,RDH11,AKR1C3,DHRS3,RDH5,RDH1,ADH4 |
| protein heterodimerization activity | H2AFB2,TAF13,YWHAE,TBX15,HIST1H2B8,CEBPG,ABTB2,IL12A,SOX5,TAF9B |
Interacting Protein
Bmp2 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with Bmp2 here. Most of them are supplied by our site. Hope this information will be useful for your research of Bmp2.
BMPR1A;FSTL1;BMPR1B;NOG;WFIKKN2
Resources
-

Revolutionize Your Research with Our Superior BMP2 Recombinant Protein
-

Specialized Cytokines for Organoid Culture — Robust Liver Organoid Culture Solutions
Gene Families
Related Services
Related Products
References
- Olivares-Navarrete, R; Hyzy, SL; et al. Coordinated regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by endogenous bone morphogenetic proteins. BONE 73:208-216(2015).
- Xu, LL; Liu, Y; et al. U0126 promotes osteogenesis of rat bone-marrow-derived mesenchymal stem cells by activating BMP/Smad signaling pathway. CELL AND TISSUE RESEARCH 359:537-545(2015).




