Hemangioblast Markers
Related Symbol Search List
Immunology Background
Hemangioblast
Hemangioblasts (Hematopoietic Stem Cells) are primitive progenitor cells that give rise to both hematopoietic and endothelial cells. The concept of hemangioblasts dates back to the early 20th century when Florence Sabin first proposed the idea of a common precursor for blood and blood vessels. However, it wasn't until the advent of advanced molecular and cellular techniques that the existence and characteristics of hemangioblasts were more clearly defined.
Hemangioblasts emerge during the early stages of embryogenesis, specifically within the mesoderm, a middle layer of the embryo that also gives rise to muscle, bone, and connective tissue. These progenitor cells are primarily identified during the gastrulation phase, where the three germ layers (ectoderm, mesoderm, and endoderm) are formed. Hemangioblasts are crucial for the formation of the blood islands in the yolk sac, which are early sites of blood and blood vessel formation in the embryo.
Hemangioblasts are bipotent progenitor cells, meaning that have the potential to differentiate into two distinct lineages: hematopoietic cells (which form blood cells) and endothelial cells (which form blood vessels). The differentiation of hemangioblasts into these lineages involves a complex interplay of genetic and environmental factors. Hemangioblasts give rise to hematopoietic stem cells, which are multipotent cells capable of generating all blood cell types, including red blood cells, white blood cells, and platelets. Key transcription factors and signaling molecules, such as Runx1, Gata2, and Scl/Tal1, play crucial roles in driving the differentiation of hemangioblasts into hematopoietic stem cells. The transition to Hematopoietic Stem Cells (HSCs) involves a series of intermediate progenitors, each progressively restricted in their differentiation potential.
Hemangioblasts also differentiate into endothelial cells, which line the interior surface of blood vessels, including arteries, veins, and capillaries. The formation of endothelial cells from hemangioblasts is essential for vasculogenesis (the de novo formation of blood vessels during embryogenesis) and angiogenesis (the sprouting of new blood vessels from pre-existing ones). Flk-1 (VEGFR-2) is a critical marker and regulator of this process, mediating responses to VEGF (Vascular Endothelial Growth Factor) signaling.
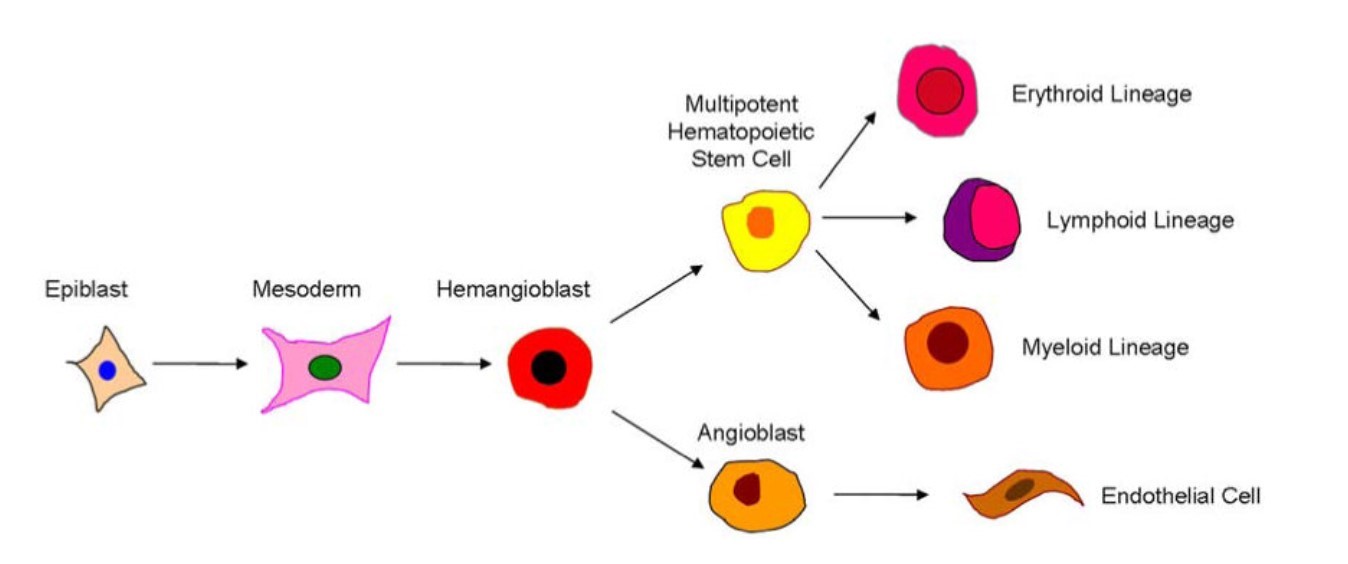 Fig. 1: Schematic diagram showing the purported derivation of endothelial and hematopoietic cells (Psaltis et al., 2010).
Fig. 1: Schematic diagram showing the purported derivation of endothelial and hematopoietic cells (Psaltis et al., 2010).Background
To identify and study hemangioblasts, specific markers are needed to differentiate these progenitors from other cell types. Hemangioblast markers thus serve as important tools in the study of early development, disease modeling, and potential therapeutic applications.
Definition and Importance of Hemangioblast Markers
Markers are specific proteins, genes, or other molecular features that allow researchers to identify and isolate particular cell types. Hemangioblast markers are therefore crucial for distinguishing hemangioblasts from other progenitor cells and for studying their properties, lineage potential, and differentiation pathways. These markers play an essential role in understanding the developmental origins of blood and blood vessels and are invaluable in regenerative medicine and therapeutic research.
Markers can be cell surface proteins, transcription factors, or specific genes expressed in hemangioblasts. Identification of these markers allows researchers to track the presence and differentiation of hemangioblasts in various biological contexts, including embryonic development, disease states, and in vitro differentiation systems.
Examples of Hemangioblast Markers
Several markers have been identified as indicative of hemangioblasts. These markers help to characterize and isolate hemangioblasts and to understand their differentiation into hematopoietic and endothelial lineages.
Stem Cell Leukemia (Scl), also known as T-cell Acute Lymphocytic Leukemia Protein 1 (Tal1), is a transcription factor essential for hematopoietic and endothelial development. Scl/Tal1 is expressed in hemangioblasts and is crucial for their differentiation into hematopoietic cells. Knockout studies have shown that the absence of Scl/Tal1 leads to the failure of blood and blood vessel formation, underscoring its importance as a hemangioblast marker.
CD34 is a cell surface glycoprotein commonly used as a marker for hematopoietic stem cells and progenitor cells. This protein is expressed on the surface of hemangioblasts and other progenitor cells. Its presence helps to identify hemangioblasts in early embryonic tissues and in hematopoietic differentiation assays.
Runt-related transcription factor 1 (RUNX1) is a transcription factor essential for the establishment of definitive hematopoiesis. RUNX1 expression is critical for the transition of hemangioblasts to hematopoietic stem cells. It plays an important role in the definitive hematopoietic system, which is responsible for generating all blood cell types throughout the life of an organism.
CD31, also known as Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1), is an integral membrane protein expressed on endothelial cells and hematopoietic cells. CD31 is used to identify endothelial progenitor cells derived from hemangioblasts. Its presence on hemangioblasts indicates their potential to differentiate into endothelial cells and contribute to the formation of the vasculature.
Applications of Hemangioblast Markers
The identification and study of hemangioblast markers has several important applications in biological research and medicine:
Developmental Biology
Study of hemangioblast markers helps to understand early development. Hemangioblast markers are essential tools for studying the early stages of blood and vessel formation. They allow researchers to trace the lineage and differentiation pathways of these progenitor cells, providing insight into the molecular mechanisms that control embryogenesis.
Regenerative Medicine
- Stem Cell Therapy: Hemangioblast markers are critical for isolating and characterizing progenitor cells for potential therapeutic applications. These markers enable the development of stem cell-based therapies for diseases such as anemia, leukemia, and vascular disorders.
- Tissue Engineering: By identifying and manipulating hemangioblasts, researchers can create engineered tissues with functional blood vessels, improving the success of tissue grafts and implants.
Disease Modeling
Hemangioblast markers help to model diseases involving blood and blood vessel formation, such as congenital anemia, hemangiomas, and vascular malformations. These models are crucial for studying disease pathogenesis and testing potential treatments.
Hemangioblast markers are used in high-throughput screening assays to identify compounds that affect blood and blood vessel formation. These assays are critical to the discovery of new drugs for hematologic and vascular diseases.
Case Study
Case 1: Silva, A.; et al. Colon tumor CD31 expression is associated with higher disease-free survival in patients with metabolic syndrome. Pathol Res Pract. 2022 Dec;240:154182.
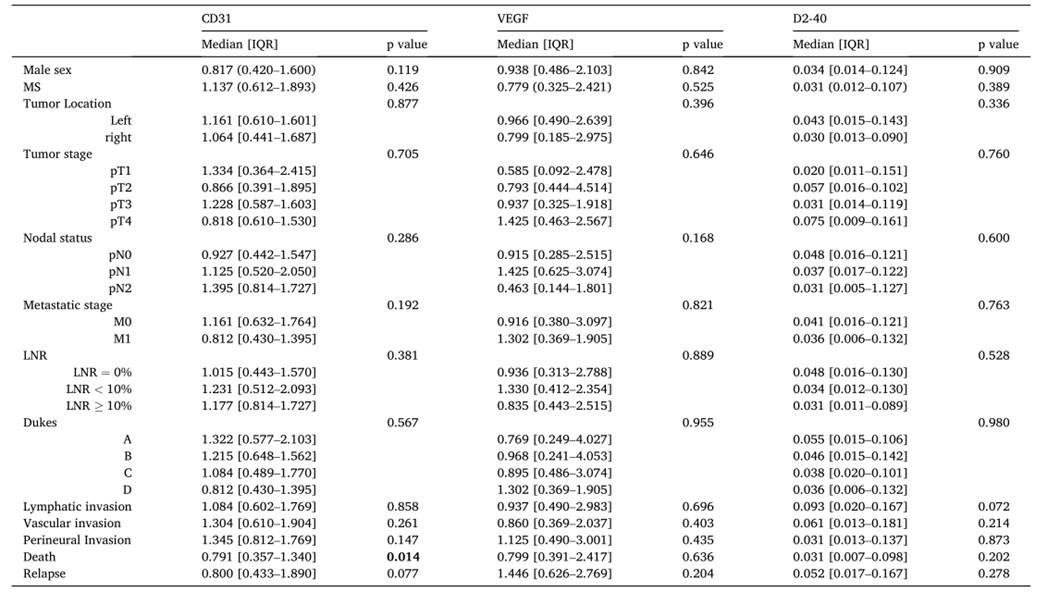
Metabolic syndrome (MS) is recognized as a risk factor for colon cancer (CC). However, how does the interplay between metabolic dysfunction caused by MS and its individual components affect CC microenvironment and prognosis remains unexplored. In this study, Thus, the researchers evaluated whether the expression of molecular biomarkers involved in angiogenic and lymphangiogenic processes influenced CC clinicopathological features and prognosis in patients with MS. The results demonstrated that CD31 expression was significantly associated with greater disease-free survival. In conclusion, they report that suggest the potential use of CD31 as a CC prognostic biomarker.
Tab.1: Association between expression of CD31, VEGF and D2–40 and clinical data of patients included in the study (n = 102).
Case 2: Seco, Pedro; et al. A bird's eye view on the origin of aortic hemogenic endothelial cells. Frontiers in Cell and Developmental Biology, vol. 8, Nov. 2020, p. 605274.
During early embryogenesis, the hemogenic endothelium of the developing dorsal aorta serves as the primary source of the definitive hematopoietic stem cells (HSCs) responsible for the generation of all blood cell lineages in the adult organism. These hemogenic endothelial cells (HECs) are derived from the splanchnic lateral plate mesoderm. However, the precise cell lineages and developmental pathways that give rise to aortic HECs remain unclear. For the past fifty years, the scientific community has debated the origin of aortic HECs and HSCs, focusing on two potential and seemingly alternative sources: the extraembryonic yolk sac blood islands and the intraembryonic splanchnic mesoderm. The researchers propose that both yolk sac blood islands and aortic HECs may share a common hemangioblastic origin.
Their studies suggests that hemangioblasts colonize the presumptive dorsal aorta region (stages HH7–8), contribute to the developing aortic endothelium (stages HH9– 10) and give rise to aortic HECs (stage HH11 onwards).
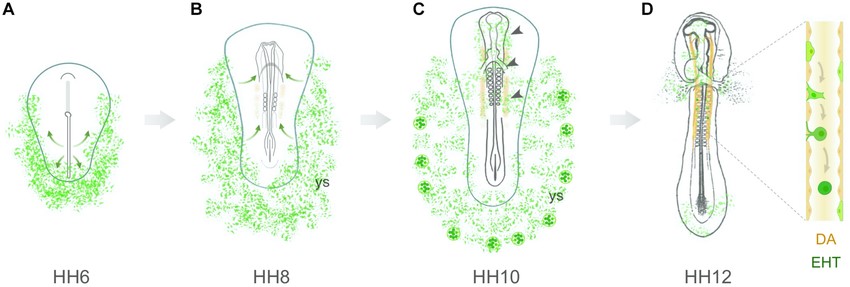 Fig. 2: Hemangioblastic origin of aortic hemogenic endothelial cells in the early chick embryo. (A) Hemangioblasts arise from lateral plate mesodermal cells that ingress through the posterior region of the primitive streak. At stage HH6, these cells are located in the lateral and posterior regions of the embryo, both in the yolk sac and intraembryonic region. (B) At stage HH7–8, hemangioblasts aggregate to form blood islands within the yolk sac (ys). Concomitantly, a subpopulation of hemangioblast-derived cells (or angioblasts) starts to migrate toward the presumptive paired dorsal aortae (orange). (C) At stage HH10, yolk sac hemangioblasts have already started to differentiate into endothelial and hematopoietic cells in the distal blood islands. Inside the embryo, hemangioblast-derived cells are found in the head region, endocardium, vascular plexus and dorsal aortae (arrowheads). (D) At stages HH11–12, hemangioblast-derived HECs are detected in the dorsal aortae (DA), where they undergo an endothelial-to-hematopoietic transition (EHT).
Fig. 2: Hemangioblastic origin of aortic hemogenic endothelial cells in the early chick embryo. (A) Hemangioblasts arise from lateral plate mesodermal cells that ingress through the posterior region of the primitive streak. At stage HH6, these cells are located in the lateral and posterior regions of the embryo, both in the yolk sac and intraembryonic region. (B) At stage HH7–8, hemangioblasts aggregate to form blood islands within the yolk sac (ys). Concomitantly, a subpopulation of hemangioblast-derived cells (or angioblasts) starts to migrate toward the presumptive paired dorsal aortae (orange). (C) At stage HH10, yolk sac hemangioblasts have already started to differentiate into endothelial and hematopoietic cells in the distal blood islands. Inside the embryo, hemangioblast-derived cells are found in the head region, endocardium, vascular plexus and dorsal aortae (arrowheads). (D) At stages HH11–12, hemangioblast-derived HECs are detected in the dorsal aortae (DA), where they undergo an endothelial-to-hematopoietic transition (EHT).References
- Psaltis, Peter J., et al. "Resident Vascular Progenitor Cells—Diverse Origins, Phenotype, and Function." Journal of Cardiovascular Translational Research, vol. 4, no. 2, Apr. 2011, pp. 161–76. DOI.org (Crossref), https://doi.org/10.1007/s12265-010-9248-9.
- Seco, Pedro, et al. "A Bird's Eye View on the Origin of Aortic Hemogenic Endothelial Cells." Frontiers in Cell and Developmental Biology, vol. 8, Nov. 2020, p. 605274. DOI.org (Crossref), https://doi.org/10.3389/fcell.2020.605274.
- Silva, Ana, et al. "Colon Tumor CD31 Expression Is Associated with Higher Disease-Free Survival in Patients with Metabolic Syndrome." Pathology, Research and Practice, vol. 240, Dec. 2022, p. 154182. PubMed, https://doi.org/10.1016/j.prp.2022.154182.

