Bio-Layer Interferometry (BLI) Service
Bio-Layer Interferometry (BLI) has become one of the most versatile and efficient label-free analytical technologies for investigating biomolecular interactions. Unlike traditional biosensing systems that rely on microfluidics or complex optical components, BLI uses a simple dip-and-read format and monitors interference patterns of white light reflected from immobilized biomolecules in real time. This enables rapid, sensitive, and high-throughput characterization of binding kinetics, affinity, and specificity. Creative BioMart offers a comprehensive BLI service platform designed to support applications ranging from protein-protein interactions and antibody characterization to drug discovery, assay development, and macromolecular assembly studies, providing researchers with reliable and cost-effective solutions.
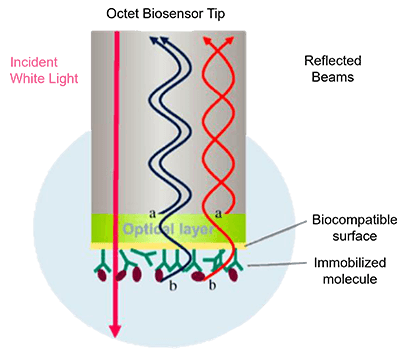
Introduction to Bio-Layer Interferometry (BLI)
Biomolecular interactions form the basis of nearly all biological pathways, influencing processes such as signal transduction, immune recognition, enzymatic activity, and cellular communication. As research moves toward increasingly complex systems, the need for highly precise and real-time characterization of these interactions continues to grow. Traditional biochemical methods, including ELISA, pull-down assays, or co-immunoprecipitation, provide valuable information but often lack the temporal resolution and kinetic detail required for advanced mechanistic studies.
Over the past decade, label-free biosensor technologies such as Surface Plasmon Resonance (SPR) and Bio-Layer Interferometry (BLI) have significantly transformed protein interaction research. Among these, BLI stands out due to its fluidics-free design, operational simplicity, and resistance to refractive index fluctuations. BLI measures the interference pattern of white light reflected from a biosensor tip onto which one binding partner is immobilized. When a second molecule binds or dissociates, the optical thickness at the biosensor surface changes, causing a measurable shift in the interference spectrum. This allows real-time quantification of association and dissociation kinetics.
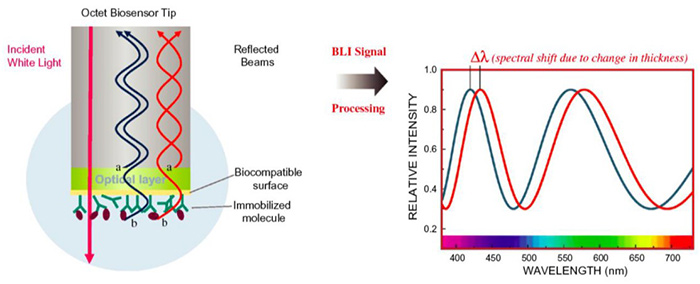
Figure 1. An optical fiber used for Bio-Layer Interferometry and a typical optical signal. (Nirschl et al., 2011)
One of BLI’s greatest advantages is its compatibility with crude samples such as serum, culture media, lysates, and complex biological matrices. Unlike methods influenced by buffer composition or refractive index changes, BLI remains stable even in heterogeneous environments. The technology is easily scalable, permitting simultaneous measurement across multiple biosensor tips without compromising accuracy or increasing procedural complexity. Duplicates, replicates, or new ligand surfaces can be created by simply immobilizing the same ligand on additional sensors, eliminating the need for complicated regeneration steps.
As BLI capabilities have expanded, so too have its applications. Today, the technology is used extensively in antibody screening, epitope mapping, fragment screening, kinetic profiling of drug–target interactions, viral particle binding studies, GPCR interactions, quantitative protein analysis, and media or formulation development. Creative BioMart’s BLI service platform integrates advanced instrumentation, optimized sensor chemistries, and expert data interpretation to provide a complete solution for researchers across biotechnology, pharmaceuticals, academia, and diagnostics.
Bio-Layer Interferometry (BLI) Service: What We Offer
Creative BioMart provides an end-to-end Bio-Layer Interferometry service tailored to diverse experimental needs and sample types. Our offering includes:
|
Category |
Services |
|---|---|
|
Comprehensive Kinetic and Affinity Analysis |
Determination of association and dissociation rate constants (ka, kd) |
|
Calculation of binding affinity (KD) across a wide dynamic range (nM to mM) |
|
|
Full kinetic profiling for antibodies, proteins, peptides, DNA/RNA, small molecules, liposomes, and viral-like particles |
|
|
Quantitative Biomolecule Measurements |
Antibody quantitation as an ELISA alternative |
|
Protein concentration assessment in purified or crude samples |
|
|
Detection of contaminants or residual process impurities |
|
|
Screening Services |
Fragment-based small molecule screening |
|
DNA-aptamer screening |
|
|
Hit validation and secondary screening for drug discovery programs |
|
|
Inhibitor screening and potency ranking |
|
|
Assay Development and Optimization |
Customized method development |
|
Buffer and formulation optimization |
|
|
Temperature-controlled stability and interaction studies |
|
|
High-sensitivity assay design for challenging biomolecules |
|
|
Broad Sample Compatibility |
Proteins, antibodies, peptides |
|
Viral-like particles and membrane-associated complexes |
|
|
GPCR–protein interactions |
|
|
Bacterial cell–antibody interactions |
|
|
Macromolecular assemblies, liposomes, and nanoparticles |
With our dedicated team, optimized protocols, and high-throughput instrumentation, Creative BioMart provides accurate, reproducible, and publication-ready data.
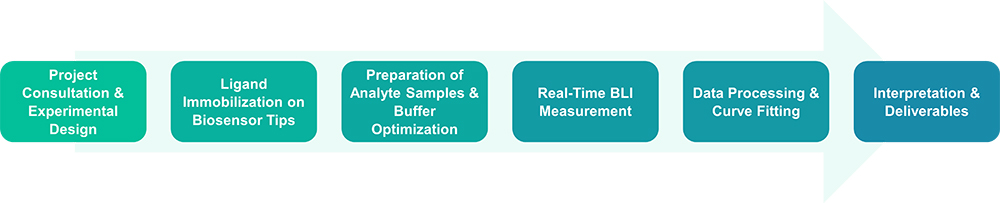
Service Workflow
Technology Advantages of BLI
Bio-Layer Interferometry offers several benefits over traditional biosensor platforms:
- Fast and easy operation with dip-and-read simplicity
- Label-free, real-time detection without fluorescent or enzymatic tags
- Complete kinetic characterization, including ka, kd, and KD analysis
- Flexible affinity range measurable from nanomolar to millimolar
- Broad buffer compatibility including detergents and solvents
- Suitability for crude samples, reducing sample preparation time
- No microfluidics, eliminating clogging risks and maintenance concerns
- High temperature flexibility, supporting interaction studies under varied conditions
- Compatibility with a wide range of ligands and molecules
- Reduced cost compared to fluidics-based kinetic platforms
Application Areas
BLI supports wide-ranging applications in pharmaceutical development, biologics optimization, and molecular research:
-
Kinetic Applications
- Protein–protein interactions
- Antibody characterization and epitope mapping
- Antigen–antibody binding
- Protein–small molecule interactions
- DNA/RNA–ligand binding
- Bacteria–antibody interactions
- Viral-like particle–protein or antibody binding
- GPCR–protein interactions
-
Quantitative Applications
- Antibody quantitation
- ELISA replacement assays
- Protein quantitation in crude extracts
- Contaminant detection
-
Screening Applications
- Aptamer screening
- Fragment-based small molecule screening
- Secondary screening and hit validation
- Inhibitor screening
-
Assay Development Applications
- Formulation development
- Culture media development
- Optimization of downstream drug development processes
Creative BioMart ensures a high level of sensitivity, reproducibility, and reliability across all these applications.
Why Choose Us
- Advanced, Fluidics-Free Kinetic Platform: Our cutting-edge BLI instruments eliminate complex microfluidic systems, reducing instrument downtime and ensuring highly stable baseline measurements.
- Superior Compatibility With Crude and Complex Samples: Unlike many biosensor technologies, BLI is resistant to refractive index changes, allowing direct measurement in serum, culture supernatants, lysates, and unpurified solutions.
- Comprehensive Expertise Across Molecular Classes: Our scientists have extensive experience in handling proteins, antibodies, peptides, nucleic acids, viral-like particles, and membrane-associated complexes.
- Tailored Experimental Design and Optimization: Each project is fully customized—from ligand immobilization strategy to buffer composition—to achieve optimal kinetic behavior and minimal non-specific interactions.
- High Throughput and Flexible Scaling: Multiple biosensors can be run in parallel, enabling efficient screening, replicates, and method development without added system complexity.
- Cost-Effective, High-Quality Data Delivery: With optimized workflows and high instrument throughput, we provide precise kinetic and affinity data at competitive pricing, making BLI a powerful alternative to traditional assays.
Bio-Layer Interferometry (BLI) Service: Case Studies
Case 1: BLI support rational antibody humanization
Margreitter et al., 2016. doi:10.1002/jmr.2527
This study showcases how Bio-Layer Interferometry (BLI) can support rational antibody humanization by rapidly assessing binding affinities of engineered monoclonal antibody variants. Researchers combined molecular dynamics–based predictions with BLI measurements to evaluate backmutations in the model antibody Ab2/3H6. Using streptavidin and protein A biosensors, they quantified association and dissociation kinetics from both purified antibodies and crude culture supernatants, enabling high-throughput screening without requiring antigen–antibody structural data. BLI reliably distinguished strong binders from non-binders and produced consistent affinity estimates, which were converted into binding free energies for comparison with simulation-derived scores. This integrative workflow accelerates identification of optimized humanized antibody candidates.
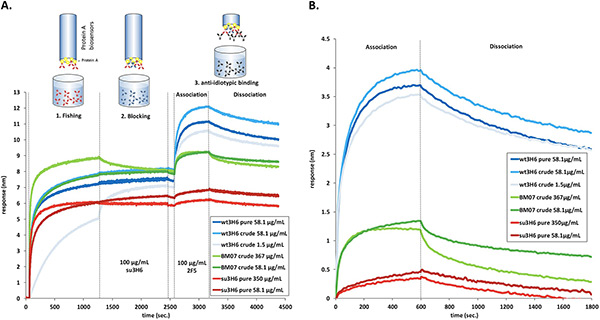
Figure 2. Bio-layer interferometry protein A fishing of Ab2/3H6 variants from crude supernatants using ForteBio Octet. (A) Real-time sensorgrams showing immobilization, blocking, association with mAb 2F5, and dissociation. (B) Processed association and dissociation curves aligned to baseline. (Margreitter et al., 2016)
Case 2: Application of bio-layer interferometry for the analysis of protein/liposome interactions
Wallner et al., 2013. doi:10.1016/j.jpba.2012.10.008
This study demonstrates how Bio-Layer Interferometry (BLI) can be adapted to analyze protein–liposome interactions with high sensitivity and throughput. Using recombinant human erythropoietin immobilized on streptavidin biosensors, researchers measured the binding behavior of three liposome formulations (DPPC, egg-PC, POPC). Optimized loading, equilibration, association, and dissociation steps produced reproducible kinetic profiles. All liposomes formed stable complexes, but with clear lipid-dependent affinity differences: DPPC showed the strongest binding, followed by egg-PC and POPC. Responses were also concentration-dependent across a defined 2.5–10 mM range. The method’s simplicity, automation compatibility, and robust kinetics make it valuable for studying drug–membrane interactions and early-stage screening.
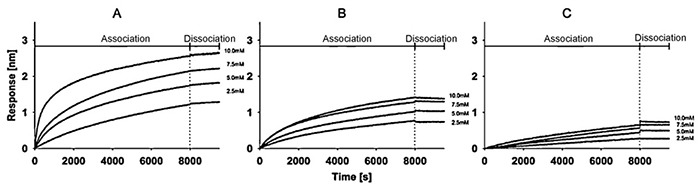
Figure 3. Association and dissociation curves of DPPC (A), egg-PC (B) and POPC (C) liposomes in a concentration range between 10 mM and 2.5 mM. B-rh-Epo was immobilized on Streptavidin sensor tips prior association. (Wallner et al., 2013)
Bio-Layer Interferometry (BLI) Service: Customer Feedback
“We engaged Creative BioMart to perform high-throughput BLI characterization on a panel of humanized antibody variants targeting a challenging cytokine receptor. Their team delivered precise KD, ka, and kd measurements even from partially purified culture supernatants, saving us weeks of upstream prep. Their rapid turnaround enabled us to confidently prioritize three leads with sub-nanomolar affinities for IND-enabling studies. The clarity of their data presentation and the soundness of their experimental design made internal review a breeze.”
— Director of Antibody Discovery | Global Biopharmaceutical Company
“We needed accurate kinetic profiles for a library of engineered peptide inhibitors binding to a membrane-associated kinase domain. Creative BioMart optimized sensor selection, immobilization conditions, and buffer composition to avoid nonspecific binding that had stalled our internal work. Their BLI datasets were not only clean and reproducible but also provided mechanistic insights into multiple binding modes. The report included detailed association/dissociation curve fitting and comparative affinity tables, which directly guided our SAR decisions.”
— Principal Scientist, Protein Sciences | Mid-Size Biotechnology Firm
“Our group required a robust BLI-based quantitation assay to monitor antibody titer shifts across a series of fed-batch process optimization runs. Creative BioMart developed a fully validated assay using anti-Fc sensors, demonstrating strong linearity and cross-matrix consistency between clarified harvest and crude lysates. Their method reduced our dependency on ELISA and improved our ability to make real-time process decisions. The scalability of their BLI workflow fit perfectly with our high-throughput development model.”
— Head of Analytical Development | Contract Development & Manufacturing Organization (CDMO)
“For a collaborative drug discovery project, we needed secondary screening to validate fragment hits identified from an in-house NMR screen. Creative BioMart used their BLI system to evaluate over 120 fragments against a recombinant GPCR extracellular domain. The sensitivity of their setup allowed detection of weak-affinity binders in the high-micromolar range, which was essential for fragment progression. Their detailed analysis—including sensorgram overlays, Rmax comparisons, and hit categorization—significantly strengthened the dataset we provided to our medicinal chemistry partners.”
— Director of Molecular Screening | University Core Research Facility
Bio-Layer Interferometry (BLI) Service: Frequently Asked Questions (FAQs)
-
Q: What types of biomolecular interactions can your BLI platform analyze?
A: Our BLI service supports a wide range of interaction studies, including protein–protein, antibody–antigen, protein–small molecule, DNA–aptamer, viral-like particle binding, GPCR interactions, and more. Whether your project requires kinetic characterization, affinity ranking, quantitative analysis, or screening, our platform is optimized to deliver high-quality, label-free results across diverse molecular classes. -
Q: Can BLI measure binding kinetics using crude samples?
A: Yes. One of the major advantages of BLI is its immunity to refractive index fluctuations, making it highly suitable for crude or partially purified materials such as culture supernatants, cell lysates, or complex biological matrices. This eliminates lengthy purification steps and accelerates timelines—especially for antibody discovery and early screening programs. -
Q: How does BLI compare to other label-free platforms such as Surface Plasmon Resonance (SPR)?
A: While both SPR and BLI are powerful label-free technologies, BLI offers several practical advantages:- No microfluidics, minimizing clogging or maintenance issues.
- Simple sensor replacement, eliminating the need for surface regeneration.
- Higher throughput due to scalable sensor architecture.
- Cost-effective operation, making it ideal for larger screens and iterative studies.
-
Q: What sample amounts and purity levels are required?
A: Sample requirements depend on assay type, but BLI generally needs only small quantities (often microgram levels). Purity requirements are flexible because BLI sensors tolerate crude preparations. If needed, our scientific team will assist in optimizing concentrations, buffers, and immobilization strategies to maximize assay performance. -
Q: Do you support high-throughput screening using BLI?
A: Absolutely. Our BLI platform can be scaled to process dozens or hundreds of samples efficiently, making it ideal for fragment screening, antibody panel characterization, hit validation, and formulation and media development studies. Because each biosensor functions independently, throughput increases without increasing system complexity. -
Q: Can your team help with assay development and optimization?
A: Yes. Creative BioMart provides full assay development services, including ligand immobilization optimization, buffer selection, regeneration feasibility (if desired), sensor choice, and control strategy design. Our scientists can refine or build your BLI workflow from the ground up to ensure reliable, reproducible results. -
Q: How fast is the turnaround time for BLI projects?
A: Typical projects are completed within a short timeline due to BLI’s rapid setup and real-time detection. Most standard kinetic or quantitation studies are finalized within days after sample receipt. High-complexity or large-scale screens may require slightly longer, but our team always provides a clear schedule during project initiation.
Other Resources
Related Services
References:
- Margreitter C, Mayrhofer P, Kunert R, Oostenbrink C. Antibody humanization by molecular dynamics simulations— in‐silico guided selection of critical backmutations. J of Molecular Recognition. 2016;29(6):266-275. doi:10.1002/jmr.2527
- Nirschl M, Reuter F, Vörös J. Review of transducer principles for label-free biomolecular interaction analysis. Biosensors. 2011;1(3):70-92. doi:10.3390/bios1030070
- Wallner J, Lhota G, Jeschek D, Mader A, Vorauer-Uhl K. Application of Bio-Layer Interferometry for the analysis of protein/liposome interactions. Journal of Pharmaceutical and Biomedical Analysis. 2013;72:150-154. doi:10.1016/j.jpba.2012.10.008
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

