cGMP Cell-Based Potency Assays
Background on cGMP Cell-Based Potency Assays in Biologics Testing
Cell-based potency assays are critical analytical tools used to measure the biological activity of biopharmaceutical products. Unlike purely physicochemical tests, these assays assess a product’s functional performance in a biologically relevant system—often reflecting its mechanism of action (MOA). As such, they play a pivotal role in demonstrating product consistency, stability, and, ultimately, therapeutic efficacy.
When performed under current Good Manufacturing Practices (cGMP), cell-based potency assays meet the rigorous standards required for lot release, stability testing, and regulatory submissions. Regulatory agencies such as the FDA and EMA emphasize that potency assays must be quantitative, reliable, and MOA-reflective, and should be validated to ensure accuracy, precision, linearity, and robustness.
Developing and validating a cGMP-Compliant Cell-Based Potency Assay requires deep scientific understanding, optimized cell lines, and strict quality control procedures. Creative BioMart brings extensive expertise across every stage of bioassay development—from assay design and validation to method transfer and long-term maintenance. We offer a dedicated, turnkey solution tailored to your cGMP cell-based potency assay needs.
Our Comprehensive cGMP Cell-Based Potency Assay Services
Service Procedure

Service Items
|
Item |
Description |
|---|---|
|
Cell Migration Assays |
Quantify and monitor cell motility in response to therapeutic agents, ideal for angiogenesis, wound healing, and cancer metastasis studies. |
|
Cell Proliferation & Inhibition Assays |
Measure stimulation or suppression of cell growth using metabolic or DNA-based readouts—critical for growth factors, antibodies, and oncology products. |
|
Apoptosis & Cell Death Assays |
Evaluate drug-induced programmed cell death via caspase activation, membrane integrity, or annexin V binding under GMP-compliant conditions. |
|
Cytotoxicity Assays |
Assess compound-induced cell damage or lysis using luminescent or colorimetric detection methods—essential for biologics, ADCs, and immunotherapies. |
|
Metabolic Activity Assays |
Monitor changes in cellular metabolism, including ATP production and mitochondrial function, to reflect drug-induced bioenergetic responses. |
|
Ligand and Receptor Binding Assays |
Quantify drug-target interactions, including affinity and specificity, via radioligand, ELISA, or fluorescence-based formats. |
|
Cell Signaling Pathway Assays |
Measure activation or inhibition of intracellular signaling cascades (e.g., MAPK, NF-κB) to confirm mechanism-of-action at the molecular level. |
|
Competitive Binding Assays |
Evaluate inhibitory effects of test compounds by displacing labeled ligands from target receptors in a dose-dependent manner. |
|
Cytokine & Metabolite Production Assays |
Quantify release or potentiation of cytokines, chemokines, or metabolites in response to drug treatment using multiplexed or single-analyte assays. |
|
Transfection & Transduction Efficiency Assays |
Measure gene delivery efficiency using reporter constructs or flow cytometry—essential for gene therapy and viral vector testing. |
|
Receptor Gene Expression Assays |
Assess upregulation or downregulation of receptor genes through qPCR, reporter activity, or surface expression analysis. |
|
Subcellular Localization Assays |
Track protein or compound distribution within cellular compartments via fluorescent tagging, confocal microscopy, or image-based cytometry. |
Why Our cGMP Potency Assays Ensure Regulatory Confidence
- Efficiency and Scalability: Projects are executed with agile timelines and a scalable operational infrastructure, enabling efficient handling of studies of varying scope and complexity.
- Regulatory-Ready Validation: All assays are developed and validated in alignment with FDA, EMA, and ICH guidelines, ensuring full regulatory compliance and audit readiness.
- Mechanism-of-Action Reflective Design: Assays are biologically relevant and MOA-aligned, providing accurate insight into therapeutic function and supporting product characterization and release.
- Comprehensive Assay Platforms: We support diverse formats—from reporter gene and cytotoxicity assays to cytokine profiling—covering a wide range of biopharmaceutical products.
- Dedicated Scientific Support: Our expert scientists provide end-to-end support, including assay design, troubleshooting, data interpretation, and regulatory guidance.
- GMP-Compliant Infrastructure: All services are performed in GMP-certified labs with strict quality control, documentation, and traceability across every phase of the project.
Case Studies on cGMP Cell-Based Potency Assays
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Nanofibrous materials affect the reaction of cytotoxicity assays
Podgórski et al., 2022. doi:10.1038/s41598-022-13002-w
Nanofibrous materials, often explored as extracellular matrix substitutes, must undergo thorough safety evaluations, including cytotoxicity testing. This study examined inconsistencies in widely used metabolic cytotoxicity assays when applied to nanofibrous scaffolds made from poly-L-lactic acid, polyurethane, and polycaprolactone. Despite similar physical properties, the same materials yielded varying cell viability results across multiple assays, indicating that nanofibers can influence assay reactions. However, all materials tested met ISO EN 10993–5 standards and showed no cytotoxicity. The findings highlight the need for validating cytotoxicity assays with nanofibrous materials using complementary tools like confocal microscopy.
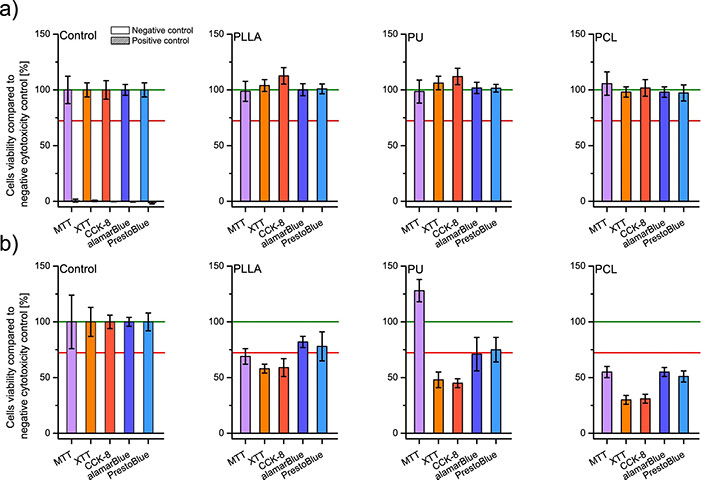
Figure 1. Cells viability was tested by five assays (a) on scaffolds' liquid extracts after 24 h of incubation with PLLA, PU, and PCL; and (b) after 24 h of direct culture on PLLA, PU, and PCL scaffolds; including controls. In every graph, the green line represents the negative control result, and a red line represents the threshold for cytotoxicity level of 70% of cells viability compared to the negative control. (Podgórski et al., 2022)
Case 2: Resveratrol strengthens cisplatin’s inhibition of TNBC cell growth and migration
Yang et al., 2021. doi:10.3390/molecules26082204
This study explored the synergistic anti-cancer effects of resveratrol combined with cisplatin on triple-negative breast cancer (TNBC) MDA-MB-231 cells. In vitro, the combination significantly reduced cell viability in a dose-dependent manner and inhibited TGF-β1-induced migration and invasion, as shown by MTS and Transwell assays. Resveratrol also modulated EMT markers by upregulating E-cadherin and downregulating vimentin, fibronectin, and key signaling proteins like P-AKT, P-PI3K, P-JNK, and P-ERK. In vivo, the combination enhanced tumor suppression while mitigating cisplatin-induced toxicity in mice. These effects are likely mediated through the PI3K/AKT, JNK, ERK, and NF-κB pathways, highlighting a promising therapeutic strategy for TNBC.
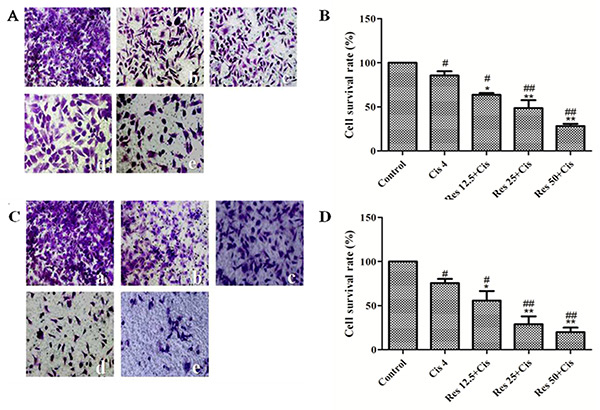
Figure 2. Effects of resveratrol combined with cisplatin on the migration and invasion of MDA231 cells. Transwell chamber migration and invasion experiments (×200). (A) Transwell chambers images of cell migration; (B) cell migration rate; (C) transwell chambers images of cell invasion; (D) cell invasion rate. (a) Control group; (b) 4 μM cisplatin group; (c) 12.5 μM resveratrol + 4 μM cisplatin group; (d) 25 μM resveratrol + 4 μM cisplatin group; (e) 50 μM resveratrol + 4 μM cisplatin group. # vs. control group; * vs. cisplatin group. (Yang et al., 2021)
Client Experiences with Our cGMP Potency Assay Services
“We required a validated cell proliferation assay to measure the potency of our monoclonal antibody for oncology indications. Creative BioMart’s expertise in assay optimization and GMP-compliant validation ensured we met stringent regulatory requirements without delays.”
— R&D Director | Global Pharmaceutical Company
“Creative BioMart developed a receptor-binding competitive assay critical for our biosimilar development program. Their team’s insight into assay linearity and robustness significantly accelerated our IND filing timeline.”
— Senior Scientist | Mid-Sized Biosimilar Manufacturer
“We needed high-throughput cytokine profiling for our lead compound targeting rheumatoid arthritis. Creative BioMart delivered not only a robust multiplex assay but also helped us interpret subtle changes in IL-17 and IFN-γ levels that correlated with patient response. Their ability to fine-tune the assay to our needs saved us weeks of optimization.”
— Senior Scientist | Biotech Startup Focused on Autoimmune Therapies
“The apoptosis and cytotoxicity assays designed by Creative BioMart gave us critical insights into our ADC candidate’s mechanism of action. Their rapid turnaround and detailed validation reports made them a trusted partner for our lot release testing.”
— Director of Preclinical Development | Biologics CDMO
FAQs on Cell-Based Potency Assays
-
Q: How do you ensure cGMP compliance throughout the potency assay process?
A: We perform all assay development, validation, and sample testing in GMP-certified labs with strict documentation, quality control, and traceability to meet regulatory standards. -
Q: Can you customize potency assays to reflect the specific mechanism of action of our biologic?
A: Yes! We tailor assay formats—whether proliferation, cytotoxicity, receptor-binding, or signaling—to closely mimic your product’s biological activity and therapeutic function. -
Q: How quickly can you develop and validate a cGMP potency assay?
A: Timelines vary by assay complexity, but our streamlined workflows and experienced team enable accelerated development and validation without compromising quality. -
Q: Do you provide ongoing support after assay validation?
A: Absolutely. We offer method transfer, long-term assay maintenance, troubleshooting, and bridging studies to support your product’s lifecycle and commercial manufacturing needs.
Resources
Related Services
- Enzyme Activity Assay
- Bioanalytical Assay Services
- Protein Methylation Assay
- Protein Acetylation Assay
- Protein Deubiquitination Assay
- Metabolism Assays
- Protein Pathway Profiling
- Protein Binding Site Mapping
- Cytokine and Receptor Analysis
- Protein Expression Microarray
- Intracellular Cytokine Detection and Activity Assay
- Ligand-Protein Interactions Screening Based on Gold Nanoparticles
Related Products
References:
- Podgórski R, Wojasiński M, Ciach T. Nanofibrous materials affect the reaction of cytotoxicity assays. Sci Rep. 2022;12(1):9047. doi:10.1038/s41598-022-13002-w
- Yang MD, Sun Y, Zhou WJ, et al. Resveratrol enhances inhibition effects of cisplatin on cell migration and invasion and tumor growth in breast cancer mda-mb-231 cell models in vivo and in vitro. Molecules. 2021;26(8):2204. doi:10.3390/molecules26082204
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

