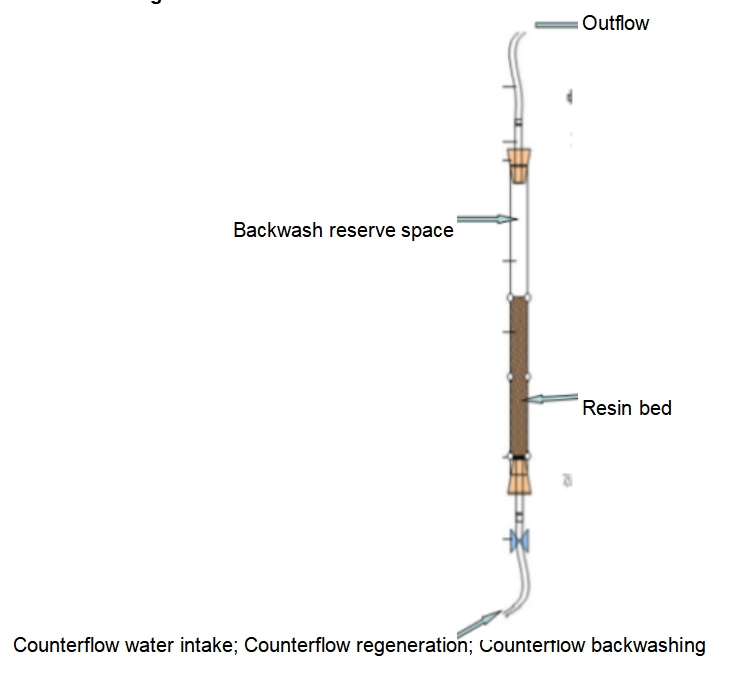
Adsorption Chromatography
Background
Overview
Adsorption Chromatography is a liquid chromatography technique that is based on the adsorption difference between the sample molecule and the stationary phase to achieve separation. In adsorption chromatography, the stationary phase is usually a solid material with adsorption properties, such as silica gel, alumina, or activated carbon. When a mobile phase (solvent or buffer) passes through a column containing a stationary phase, different molecules in the sample will remain in the column for different times due to different adsorption forces with the stationary phase, thus enabling separation.
Advantages and Disadvantages of Adsorption Chromatography
Advantages:
Simple and easy, the cost is relatively low.
Suitable for many types of samples, including non-polar and polar compounds.
Better separation results can be achieved, especially when separating the active ingredients in complex mixtures.

Cons:
There may be irreversible adsorption of sample molecules with stationary phase, resulting in sample loss.
The stability and repeatability of the column may be affected by many factors, and the experimental conditions need to be carefully controlled.
For some very polar or hydrophobic molecules, mobile phase conditions may need to be optimized for effective separation.
Influence Factors
Adsorption chromatography is a commonly used chromatography technology, and its separation effect is affected by many factors. The following are some of the main factors that affect the performance of adsorption chromatography:
The adsorbent. The adsorbent is one of the important factors affecting the effect of adsorption chromatography. Commonly used adsorbents include silica gel, alumina, etc., which have a high surface area and porosity, and can adsorb different compounds through intermolecular forces. The adsorption capacity of different adsorbents to compounds is different, so choosing the right adsorbents is very important to improve the separation effect.
The solvent. The choice of solvent also has significant influence on the separation effect of adsorption chromatography. The polarity, pH value and ionic strength of the solvent all affect the adsorption and desorption behavior of the compound on the adsorbent. By adjusting the composition and proportion of the solvent, the separation effect and retention time can be optimized.
The column. The length and diameter of the column and the particle size of the packing will affect the separation effect. In general, longer columns and smaller packing sizes provide better separation results, but may also result in higher column pressure and longer analysis times.
The temperature. Temperature control in the chromatography process is also important. The increase in temperature usually increases the diffusion rate of the compound, which affects its retention time on the column and the separation effect.
Flow rate. The flow rate of the mobile phase affects the retention time of the compound on the column. Too fast a flow rate may result in inadequate separation, while too slow a flow rate may increase the analysis time.
Differences in Various Chromatographic Techniques
From the fundamentals, the adsorption chromatography relies on physical adsorption between sample components and stationary phases (usually solid adsorbents) for separation. Different compounds have different adsorption strengths on the adsorbent surface, causing them to move at different speeds in the column, thus achieving separation. Other chromatographic techniques may use the difference in the partition coefficient between the fixed and mobile phases of the sample components, according to the molecular size or molecular volume, the charge interaction between the sample components and the ions on the stationary phase, etc.
From the stationary phase, the stationary phase of adsorption chromatography is usually a porous solid material with a high specific surface area, such as silica gel, alumina, etc. Stationary phases for other chromatographic techniques may be liquid coatings, ion exchange resins, gel beads, or specific biomolecules.
In terms of the experimental environment, adsorption chromatography is usually performed at room temperature and does not require special temperature control. Other chromatography techniques, such as gel permeation chromatography, may require operation under specific temperature conditions to maintain the structural stability of the gel.
Commonly Used Adsorbent
Adsorption chromatography is a chromatographic technique that uses the difference in adsorption capacity of adsorbents to separate different components. In adsorption chromatography, the commonly used adsorbents include activated carbon, silica gel, alumina and molecular sieve. The following are the characteristics of these adsorbents:
Activated carbon is usually made from wood, fruit shells, coal and other carbon-containing raw materials through the carbonization and activation process. It has a very high specific surface area and porous structure, so it has a strong adsorption capacity. Activated carbon has a good adsorption effect on non-polar or weakly polar substances, and has good chemical stability, and is not easy to react with adsorption substances.
Silica gel is an amorphous chain and network structure of silicic acid polymer particles, usually appearing as a hydrophilic polar adsorbent. According to the pore size can be divided into coarse pore and fine pore silica gel, suitable for different adsorption needs.
Activated alumina is made by heating and dehydrating aluminum hydrate and has high activity. It has a strong affinity for water and is an adsorbent for deep drying of trace water.
Molecular sieves are adsorbents with specific pore sizes that can be screened according to molecular size. It has a selective adsorption effect on molecules of specific size and is often used for gas adsorption separation and gas drying.

Applications
Biochemistry and molecular biology. In the field of biochemistry and molecular biology, adsorption chromatography is often used to separate and purify proteins, peptides, nucleic acids, sugars and other biological macromolecules. For example, through the use of appropriate adsorbents and elution conditions, the purification of proteins can be achieved to remove salts and other small molecular impurities from the sample.
Drug analysis. In drug analysis, adsorption chromatography is used to isolate and identify drug components, drug intermediates, and drug metabolites. It can effectively help researchers analyze the purity and efficacy of drugs to ensure the safety and effectiveness of drugs.
Environmental analysis. Adsorption chromatography technology also has important applications in environmental analysis, such as the analysis of organic pollutants and heavy metal ions in water and soil. Adsorption chromatography can effectively separate and detect trace pollutants in environmental samples.
Food science. In the field of food science, adsorption chromatography can be used to detect additives, colors, flavors, and other organic compounds in food. This is of great significance for food quality control and safety assessment.
Petrochemicals. In the petrochemical industry, adsorption chromatography is used to analyze the composition of crude oil and petroleum products to help optimize refining processes and improve product quality. In addition, it can be used to monitor chemical reactions and product purity during production.
Forensic science. In forensic science, adsorption chromatography is used to analyze drugs, gunpowder residue and other chemical substances associated with crime scenes, providing important evidence for case investigation.
Case Study
Case Study 1: Aromatic polymer adsorbent resin
This study delves into the thermodynamics of liquid-phase adsorption on hypercrosslinked polystyrene networks (HPSNs), widely recognized for their distinct structure and properties. Utilizing high-performance liquid chromatography (HPLC), we have explored, for the first time, the thermodynamic intricacies of liquid-phase adsorption onto HPSNs crosslinked in the entire range of the crosslinking degree from 100 to 500%. In the case of HPSNs, the dependence of the thermodynamic characteristics of adsorption on the liquid-phase composition is different than for classical HPLC stationary phases. Moreover, the impact of the molecular structure of the studied aromatic compounds was scrutinized on the thermodynamic characteristics and selectivity of the liquid-phase adsorption on HPSNs. Investigating liquid-phase adsorption selectivity, the pivotal role of π-π interactions in separating aromatic compounds on HPSNs was demonstrated.
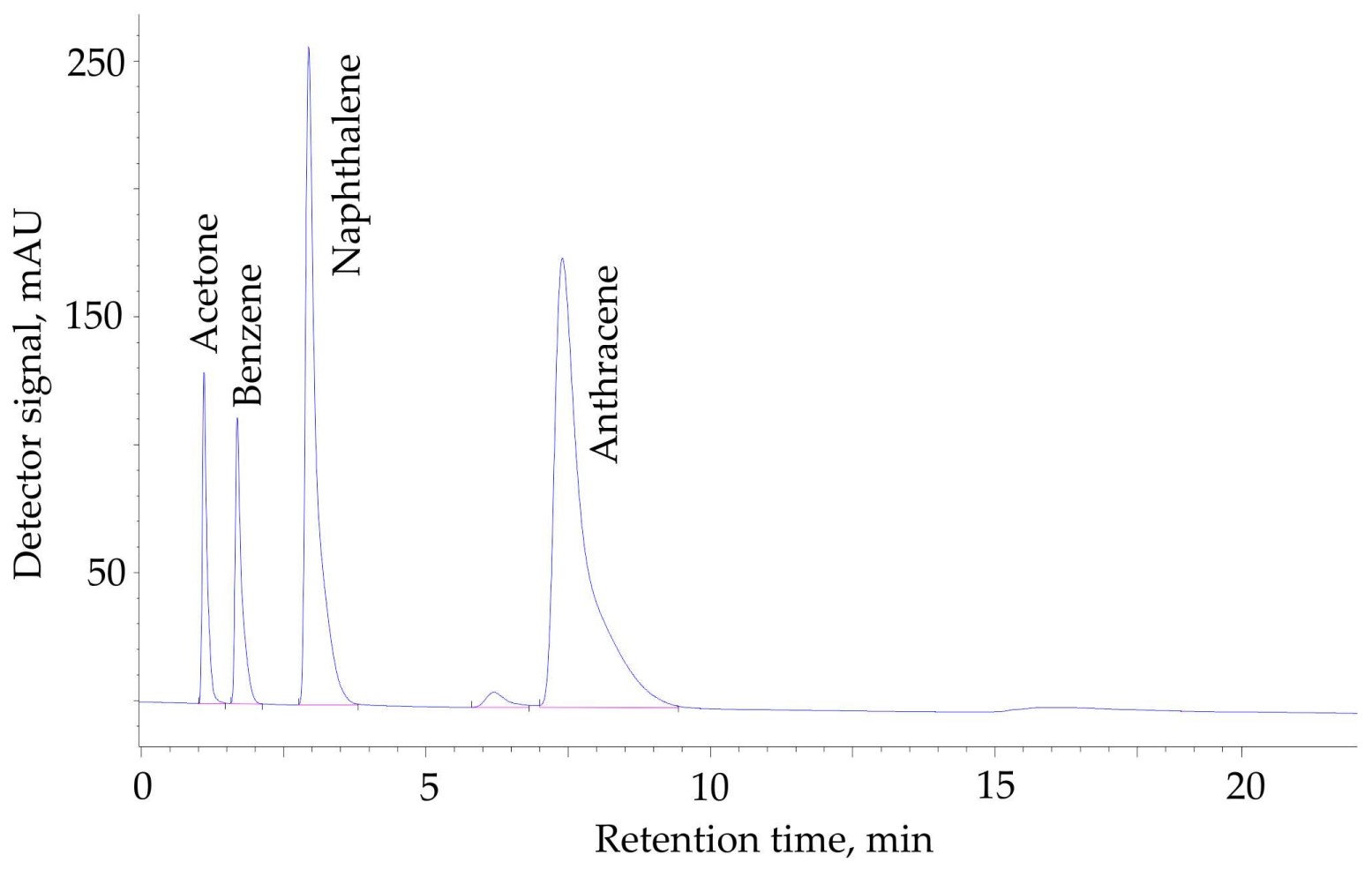
(Bulat R Saifutdinov, 2024)
Fig1. HPLC separation of acetone, benzene, naphthalene, and anthracene using the chromatographic column (50 mm × 3 mm) packed by the hypercrosslinked polystyrene with a crosslinking degree of X = 500%.
Case Study 2: Aromatic polymer adsorbent resin
Secondary varieties of date fruits have commercial value, their phytochemicals are very similar to those of the primary ones and therefore, they can be valorized as a source of compounds of interest, mainly phenols and dietary fiber. Date fruit samples were harvested at Khalal, Rutab, and Tamer stages, and a mixture of fruits from ornamental date trees was also analyzed. Aqueous and ethanolic extracts were studied for their phenolic composition. In aqueous extracts, phenols decreased with ripening, while in the ethanolic ones having higher phenolic content. Chelidonic acid, a γ-pyrone, was the major compound found in all extracts, but in the ethanolic ones, flavonoids were also present in similar amounts. After purification by adsorption chromatography, all extracts were assayed for their antimicrobial activity. Those from the Tamer stage showed the highest activity, especially against Gram-positive bacteria. The fibrous residues after aqueous and ethanolic extractions were also characterized. Their chemical composition suggested that they can be considered as a good source of prebiotic arabinoxylans and antioxidant fiber, whose antiradical activity correlated with their phenolic content.
(Amel Hamdi, 2023)
Fig2. Recoveries (%) and distribution of the different bioactive compounds after adsorption chromatography.
Case Study 3: Flash Chromatography
The purpose of this work was to investigate the bioactive compounds in T. orientalis leaf extract and their cytotoxicity in the BF-2 fish cell line (ATCC CCL-91). Flash column chromatography was used to produce 25 mL fractions with a mixture solvent system comprised of hexane, diethyl ether, methanol, and acetone. All fractions were profiled with HPLC-DAD (mobile phase methanol:aqueous buffer, 60:40 v/v) and UV detection (wavelengths 256 and 365 nm). After drying, a yellowish powder was isolated from lipophilic leaf extract with a yield of 280 µg/g dry weight. Structure elucidation by nuclear magnetic resonance (NMR) indicated it to consist of pure β-sitosterol. The lipophilic extract and pure compound were evaluated for cytotoxicity using BF-2 cells. MTT assays showed both leaf extract and pure compound at 1 µg/mL to increase cell viability after 24 h treatment. The respective half maximal inhibitory concentration (IC50) values of leaf extract and β-sitosterol were 7,027.13 and 86.42 µg/ml, indicating a lack of toxicity in the BF-2 cell line.
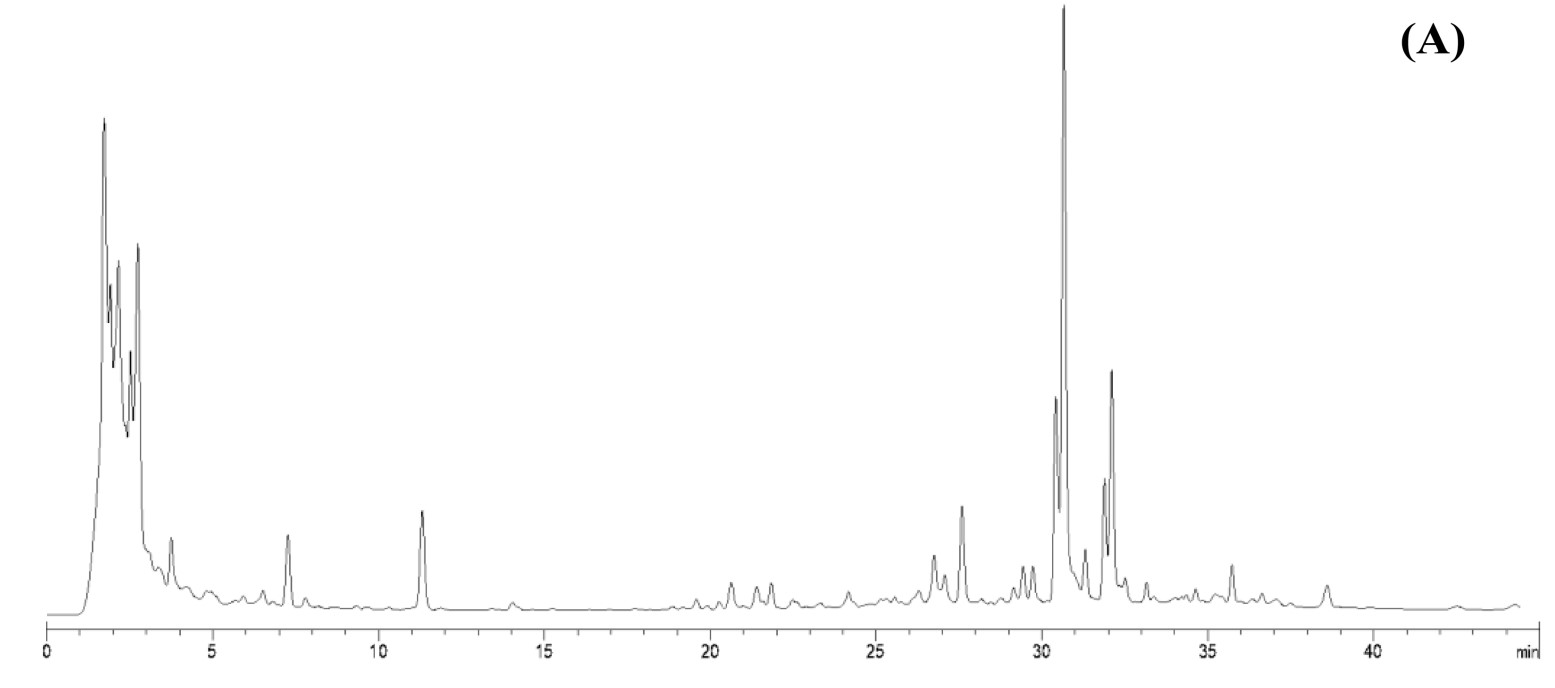
(Amita Mekarunothai, 2024)
Fig3. HPLC chromatograms of leaves lipophilic extract of T. orientalis in 10 mg/mL concentration.
Advantages
- Leading technology: We can provide a variety of adsorption chromatography technology related products, rooted in the field of chromatography, with advanced technology and research and development capabilities, can develop and optimize a variety of efficient, highly selective chromatography media.
- Wide ranges of products: We can offer different types of adsorbents such as ion exchange, metal chelation, hydrophobic interaction and affinity to meet the purification needs of different target molecules.
- Customized services: We are able to provide customized products and solutions according to the specific needs of our customers to enhance customer satisfaction and loyalty.
- Innovation ability: Continuous product development and innovation is the key to our competitiveness, being able to constantly adapt to market changes and technological advances.
Creative BioMart can provide different types of adsorbents such as ion exchange, metal chelation, hydrophobic interaction to meet customer’s different research goals. You can also let us know if you have any customized requirements. Please contact us for more product details.
References
- Saifutdinov BR, Buryak AK. Thermodynamic Characteristics and Selectivity of the Liquid-Phase Adsorption of Aromatic Compounds on Hypercrosslinked Polystyrene Networks with Ultimate-High Crosslinking Densities by Data of Liquid Chromatography. Int J Mol Sci. 2024;25(3):1551.
- Hamdi A.; et al. A Sustainable Approach for the Valorization of Underutilized Date Fruits. Molecules. 2023;28(15):5807.
- Mekarunothai A.; et al. β-sitosterol isolated from the leaves of Trema orientalis (Cannabaceae) promotes viability and proliferation of BF-2 cells. PeerJ. 2024;12:e16774.