Tumor Necrosis Factors (TNF) Superfamily

🧪 BMP2-01H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 283-396 aa
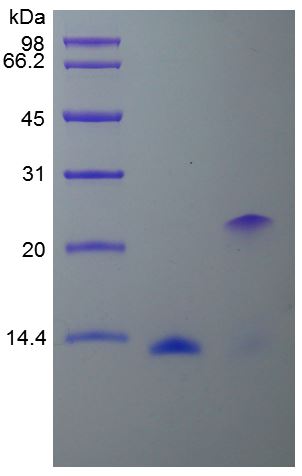
🧪 BMP5-05H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 324-454 a.a.
🧪 BMP7-10H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length:
🧪 AKT3-131H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-479 a.a.
🧪 Bmpr1a-247M
Source: HEK293
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: Met1-Arg152
🧪 BMP2-16H
Source: HEK293
Species: Human/Mouse/Rat/Rhesus/Canine
Tag: Fc
Conjugation:
Protein Length: Gln283-Arg396
🧪 BMPR2-133H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met1-Ile151
🧪 BMPR2-134H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met 1-Ile 151
🧪 Gfra1-722M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: 1-425 a.a.
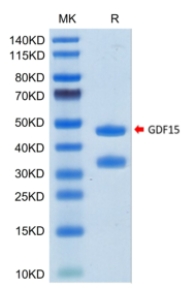
🧪 GDF15-204H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: Ala197-Ile308
🧪 TGFBR2-152H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-159 a.a.
🧪 TGFB1-126H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 279-390 a.a.

🧪 STAT3-1496H
Source: Sf9 Cells
Species: Human
Tag: GST
Conjugation:
Protein Length:

🧪 GDF15-337H
Source: CHO
Species: Human
Tag: Non
Conjugation:
Protein Length:
🧪 Bmpr1a-24M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: Met1-Arg152
TNF Superfamily—Product Overview
The Tumor Necrosis Factors (TNF) Superfamily a group of cytokines that play key roles in immune regulation, inflammation, and apoptosis. Members of this superfamily are involved in various cellular processes, including cell proliferation, differentiation, and programmed cell death. Due to their central role in immune responses and their implication in autoimmune diseases, cancer and inflammatory disorders, TNF superfamily proteins are being extensively studied for therapeutic development. Creative BioMart offers a wide range of recombinant TNF superfamily proteins and related products to support research in immunology, oncology and drug discovery.

Jump to Section
Product Families
-
TNFSF (TNF Ligands)
-
TNFRSF (TNF Receptors)
Background

The TNF superfamily is a group of structurally related cytokines and receptors that regulate immune responses, cell survival, and cell death. Most TNF ligands are type II transmembrane proteins that function as homotrimers, while their receptors—part of the TNF receptor superfamily (TNFRSF)—are type I transmembrane proteins characterized by cysteine-rich domains (CRDs) in their extracellular regions. These receptors may also contain intracellular signaling motifs, such as death domains (DDs) or TRAF binding sites, enabling them to mediate diverse downstream effects. Notable ligand-receptor pairs include TNF-α/TNFR1, FasL/Fas, TRAIL/TRAIL-Rs, and CD40L/CD40.

The TNF signaling pathways are critical regulators of immune responses, inflammation, apoptosis, and cell survival. Upon binding to TNFR1 or TNFR2, TNF triggers signaling cascades that activate NF-κB and MAPK pathways, promoting inflammation and cell proliferation. Alternatively, it can induce apoptosis through caspase activation involving adaptor proteins such as TRADD and FADD. The outcome depends on the receptor type, ligand form (membrane-bound or soluble), and cellular context. These pathways play critical roles in health and disease, particularly in cancer, autoimmunity and infectious diseases.
LEARN MORE

TNF superfamily members are key regulators of immune cell survival, differentiation, proliferation and apoptosis. They play critical roles in immune surveillance, lymphoid organ development, and defense against infection. Some members, such as FasL and TRAIL, can induce apoptosis in infected or malignant cells, while others promote cell survival and inflammation. This functional diversity is finely tuned through receptor-ligand specificity and complex signaling pathways, enabling the superfamily to maintain immune balance and tissue homeostasis.
LEARN MORE

Dysregulation of TNF superfamily signaling has been implicated in numerous diseases, including cancer, autoimmune diseases, and chronic inflammatory conditions. For example, TNF-α overexpression is associated with rheumatoid arthritis and inflammatory bowel disease, while altered Fas-FasL signaling contributes to immune evasion in tumors. Because of their dual roles in immune activation and cell death, TNF superfamily proteins are prime targets for therapeutic intervention, with several biologics in use or in development for the treatment of inflammation and cancer.
Applications

Apoptosis and Cell Death Studies
Recombinant TNF superfamily proteins such as FasL and TRAIL are commonly used to induce apoptosis in vitro, allowing researchers to study cell death pathways and screen for anti-apoptotic or pro-apoptotic drug candidates. These proteins help elucidate how cells respond to immune-mediated cytotoxicity and stress signals.
Cancer Immunotherapy Research
Native and engineered proteins of the TNF family, particularly TRAIL and CD40L, are key tools in cancer immunotherapy research. They are used to activate immune cells, induce tumor cell apoptosis, and enhance antitumor immunity in preclinical models, helping to identify new targets and develop biologic therapies.
Inflammation and Autoimmune Disease Modeling
TNF-α and other superfamily cytokines are central to the study of chronic inflammation and autoimmune diseases such as rheumatoid arthritis. Their use in cell-based assays and animal models supports the evaluation of anti-inflammatory agents and provides insight into cytokine-driven pathology.
Receptor-Ligand Interaction Analysis
Recombinant TNF superfamily proteins facilitate binding studies and high-throughput screening for agonists or antagonists targeting TNF receptors. This is essential for dissecting signaling mechanisms and for developing therapeutic agents that modulate receptor activation or inhibit pathogenic interactions.
Vaccine Development and Immune Modulation
Certain TNF family ligands, such as CD40L, are incorporated into vaccine formulations or used to prime dendritic cells in vitro. Their immunostimulatory properties enhance antigen presentation and T cell activation, making them valuable in vaccine research and immune system engineering.
Product Features
-
Comprehensive Product Range: Includes a wide selection of recombinant and native TNF superfamily proteins such as TNF-α, TRAIL, FasL, CD40L, and more—available across multiple species and isoforms.
-
High Biological Activity: Proteins are rigorously tested to ensure proper folding, receptor binding, and bioactivity, suitable for functional assays, apoptosis induction, and immune cell activation.
-
Flexible Formats: Available in carrier-free, tagged, and non-tagged versions to support diverse research needs including protein-protein interaction studies, drug screening, and cell-based assays.
-
Validated Purity and Identity: Each product undergoes strict quality control, including SDS-PAGE, HPLC, and mass spectrometry, to guarantee high purity and verified molecular identity.
-
Custom Services Available: Customized protein expression, purification, and formulation services to meet specific project requirements, including bulk orders and endotoxin-free preparations.
-
Application-Ready: Optimized for use in immunology, oncology, inflammation research, and therapeutic development, with protocols and support readily available.
Case Study
Case 1: TNF-Mediated Necroptosis in AD
Jayaraman et al., 2021. TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus.
Chronic neuroinflammation is increasingly recognized as a driver of neuronal damage in Alzheimer's disease (AD), with the TNF superfamily playing a central role. TNF signaling through TNFR1 appears to promote necroptosis rather than apoptosis in AD brains, as evidenced by increased phosphorylation of RIPK3 and MLKL, particularly in CA1 neurons. This necroptotic activity correlates with neuronal loss and shows sex-specific differences. In vitro, TNF-induced necroptosis in iPSC-derived neurons is reversible using RIPK pathway inhibitors, suggesting potential therapeutic targets.

Figure 1. TNFR1-mediated necroptosis signaling is activated in the CA1 neurons in AD hippocampus. a Analysis of mRNA levels of the TNFR1, TNFR2, and FADD genes in the hippocampus grey matter in AD cases (n = 30) and controls (n = 14). b, c Representative western blots of the soluble and membrane bound forms of TNF and TNFR1, TNFR2, and FADD and their quantification in AD (n = 15) and control cases (n = 9), normalized to GAPDH. Data are represented as mean ± SEM, *p < 0.05, ***p < 0.001.
Case 2: TRAPS Mutations Suppress TNFα
Akagi et al., 2022. TRAPS mutations in Tnfrsf1a decrease the responsiveness to TNFα via reduced cell surface expression of TNFR1.
In this study, three TRAPS mouse models carrying Tnfrsf1a mutations (T79M, G87V, T90I) were generated to study inflammatory responses at physiological levels of expression. Surprisingly, none developed spontaneous inflammation, and the T79M and G87V mutants showed reduced TNFα-induced mortality and arthritis. These mutations attenuated TNFα responses in macrophages due to decreased surface TNFR1 expression, although LPS responses remained unaffected. Overall, TRAPS mutations may suppress rather than enhance TNFα signaling, suggesting that TRAPS pathology may result from other unidentified proinflammatory triggers.
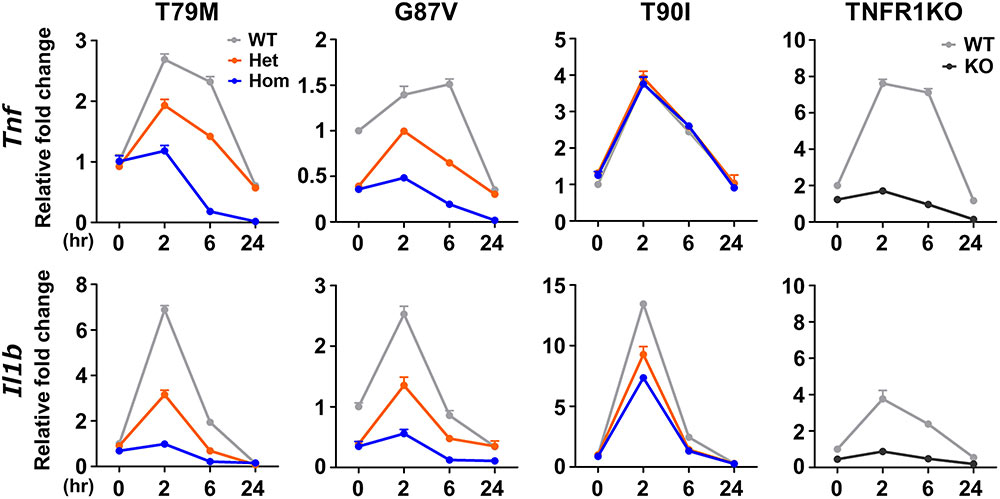
Figure 2. Decreased responsiveness to TNFα in the TRAPS mutations. Primary murine bone marrow-derived macrophages were stimulated with 100 ng/mL TNFα. RNA samples were collected at indicated time points. Cell culture experiments were performed separately for each strain. The mRNA expression levels of Tnf and Il1b were determined using qPCR. The WT levels at 0 h were set to 1. Gray, orange, blue, and dark gray lines indicate WT, heterozygous, homozygous, and TNFR1KO mice, respectively. TNFα, Tumor necrosis factor α; TRAPS, TNF receptor-associated periodic syndrome; WT, wild-type; Het, heterozygote; Hom, homozygote.
FAQs
-
Q: What types of recombinant TNF ligands and receptors do you offer?
A: We provide a diverse range of recombinant TNF superfamily ligands and receptors, including TNF-α, TNF-β, FasL, TRAIL, CD40L, and their corresponding receptors like TNFR1, TNFR2, Fas, and DR4/DR5. These proteins are available in various forms, such as His-tagged, Fc-fusion, and tag-free, to accommodate different research needs. -
Q: How are your recombinant proteins produced and purified?
A: Our recombinant proteins are produced using advanced expression systems, including E. coli, mammalian cells, and insect cells, depending on the specific protein requirements. Each protein undergoes rigorous purification processes, such as affinity chromatography and size-exclusion chromatography, ensuring high purity and activity levels.
-
Q: Are your TNF proteins biologically active?
A: Yes, our recombinant TNF ligands and receptors are tested for biological activity using relevant in vitro assays, such as apoptosis induction, NF-κB activation, or receptor-binding studies. This ensures that the proteins are functionally active and suitable for downstream applications.
-
Q: How should I store and handle these recombinant proteins?
A: Upon receipt, we recommend that the proteins be stored at -80°C for long-term storage. For short-term use, proteins should be aliquoted to avoid repeated freeze-thaw cycles, which can affect protein stability. Always refer to the specific product data sheet for detailed storage and handling instructions. -
Q: Can I request custom modifications or bulk quantities?
A: Absolutely! We offer custom services, including protein labeling (e.g., biotinylation, fluorescent tags), mutagenesis, and bulk production to meet your specific research requirements. Please contact our customer service team to discuss your project needs.

