Bioanalytical Assay Services
Creative BioMart offers comprehensive Bioanalytical Assay Services designed to deliver accurate, reproducible, and timely results across a broad range of analytes and biological matrices. Our experienced team provides complete end-to-end solutions, from sample preparation and assay execution to data analysis and report generation. We support both standard and customized protocols to meet unique research needs, ensuring the highest quality outcomes for pharmaceutical, biotechnology, academic, and clinical partners. By combining state-of-the-art instrumentation with deep scientific expertise, Creative BioMart enables clients to accelerate discovery, streamline development, and achieve robust, data-driven decisions.

Overview of Bioanalytical Assay Services

Bioanalysis is critical for understanding complex biological systems, developing therapeutic interventions, and advancing translational research. Reliable quantification of analytes such as hormones, cytokines, metabolites, and signaling molecules provides the foundation for studies in pharmacology, toxicology, and molecular biology. However, the technical demands—ranging from specialized instrumentation to assay optimization—can often exceed the resources of in-house laboratories. Creative BioMart bridges this gap by offering dedicated bioanalytical assay services. Our tailored solutions enable researchers to obtain high-quality data without the burden of equipment investment, technical training, or extensive personnel resources.
Comprehensive Bioanalytical Assay Services
Our bioanalytical assay services are designed for researchers who:
- Do not have the time or personnel to run their own assays
- Lack access to the required instrumentation
- Are unfamiliar with specific assay techniques
- Require customized or validated assay protocols
End-to-end Solutions
-

EIA/ELISAs for eicosanoids, cytokines, steroids, adipokines, oxidative stress biomarkers, and signaling molecules such as cAMP and cGMP
-
Enzyme activity assays and inhibitor screening assays for kinetic characterization, activity profiling, and drug–target interaction analysis
-
Non-EIA colorimetric and fluorometric assays for metabolites such as nitrate/nitrite, cholesterol, glutathione, triglycerides, and sphingomyelin
Our Specialized Services
We carry out a wide range of specific assays, including but not limited to:
-
Aldosterone Assay Service
-
Catalase Assay Service
-
Chloride Colorimetric Assay Service
-
Coenzyme A Assay Service
-
Colorimetric COX Inhibitor Screening Assay Service
-
cPLA2 Assay Service
-
Creatine Kinase Assay Service
-
Cysteinyl Leukotriene Assay Service
-
Glucose Assay Service
-
Glutathione Assay Service
-
Interleukin Assay Service
-
Prostaglandin Assay Service
Service Workflow

Advantages of Choosing Our Bioanalytical Testing Services
- Extensive Expertise: Our scientists are highly trained in assay design and optimization.
- Comprehensive Assay Portfolio: We provide one of the most diverse selections of bioanalytical assays available.
- Customizable Protocols: Services can be tailored to unique research needs and sample types.
- Reliable Data Quality: Every assay is performed under strict quality control for reproducibility and accuracy.
- End-to-End Solutions: From consultation to final reporting, we manage every step of the process.
- Global Trust: Trusted by academic groups, biotech startups, and leading pharmaceutical companies worldwide.
Case Studies in Bioanalytical Assays
Case 1: Characterization of the pro-inflammatory cytokine signature in severe COVID-19
Hawerkamp et al., 2023. doi:10.3389/fimmu.2023.1170012
Clinical outcomes of SARS-CoV-2 infection range from asymptomatic cases to severe pneumonia and death, largely influenced by immune response variability. In a study of 118 unvaccinated COVID-19 patients and 44 healthy controls, nearly all pro-inflammatory markers were elevated during acute infection. Eleven cytokines, including IL-1β, IL-6, IL-10, TNF-α, and IP-10, were strongly linked to disease severity. Significant correlations among markers indicated broad immune dysregulation. Principal component analysis identified a distinct pro-inflammatory cytokine signature independently associated with severe disease, need for mechanical ventilation, and mortality. These findings highlight cytokine-driven immune dysregulation as a key factor in severe COVID-19 outcomes.
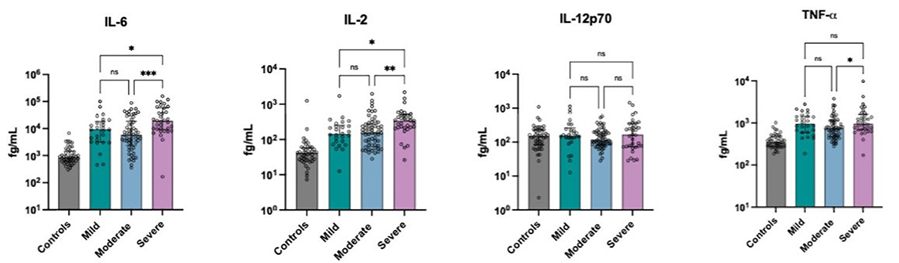
Figure 1. Cytokine concentrations in acute COVID-19. Only the IL-6 & Th1 cytokines are shown here. (Hawerkamp et al., 2023)
Case 2: Pharmacological potential of Petroselinum crispum essential oil
Foudah et al., 2022. doi:10.3390/molecules27030934
The herbal plant Petroselinum crispum (Mill), commonly known as parsley, was studied for its in vitro pharmacological activity using leaves collected from Al-Kharj, Saudi Arabia. Essential oil (PCEO) was extracted via hydrodistillation and analyzed by GC-MS, identifying 67 components that accounted for 96.02% of the oil’s composition, with myristicin (41.45%) as the dominant constituent. PCEO demonstrated strong antioxidant, antimicrobial, and anti-inflammatory activities, with notable effects against Candida albicans and Staphylococcus aureus. Complementary in silico analyses, including PASS prediction, Swiss ADME, and molecular docking, further supported its therapeutic potential, suggesting PCEO or myristicin as promising candidates for drug development.
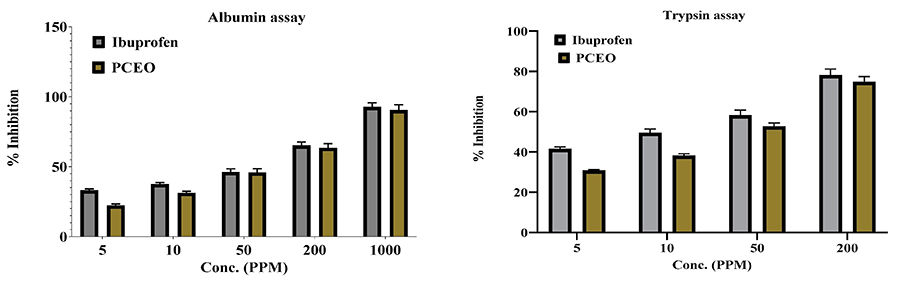
Figure 2. Anti-inflammatory activity of P. crispum leaves essential oil (PCEO). (Foudah et al., 2022)
Proven Success: Bioanalytical Assay Support Testimonials
"Our research group collaborated with Creative BioMart to profile cytokine levels in patient serum samples for an immunology study. Their team handled over 300 samples with precision, delivering consistent ELISA results even across multiple assay plates. The clear, well-documented data sets helped us identify key biomarkers of disease progression and accelerated the drafting of our manuscript. Their ability to troubleshoot cross-reactivity issues saved us weeks of optimization in-house."
— Principal Investigator | University Medical Center
"We engaged Creative BioMart to perform inhibitor screening for a novel anti-inflammatory compound targeting COX enzymes. Their assay service was fast, reproducible, and provided detailed inhibitor profiling at different concentrations. This enabled our team to make confident decisions in down-selecting candidates for preclinical testing. What stood out was their willingness to adapt the assay protocol to fit our specific molecular scaffold, something not every CRO offers."
— Director of Discovery Biology | Mid-Sized Biotech
"Our company required precise measurement of glutathione and triglycerides in plasma samples from an early-stage clinical trial. Creative BioMart delivered timely results with full quality documentation suitable for regulatory submission. Their reporting format was intuitive, allowing our clinical scientists to quickly integrate the data into our trial database. Their responsiveness during audit-related questions demonstrated professionalism and gave us confidence in their compliance standards."
— Head of Clinical Operations | Global Pharmaceutical Company
"Creative BioMart assisted us in developing a custom fluorometric assay to measure sphingomyelin levels in patient-derived fibroblast samples. Given the rarity of our disease model, off-the-shelf assays were not an option. Their scientists collaborated closely with our lab, refining the protocol and validating sensitivity against our controls. The final assay was robust and reproducible, giving us the tool we needed to push our therapeutic hypothesis forward."
— Research Director | Rare Disease Foundation
FAQs About Bioanalytical Assay Services
-
Q: What types of assays do you offer?
A: We provide a comprehensive portfolio of assays, including ELISAs for cytokines, hormones, and signaling molecules, enzyme activity assays, inhibitor screening assays, and colorimetric/fluorometric assays for metabolites such as cholesterol, glutathione, and triglycerides. We also offer specialized services for markers like aldosterone, prostaglandins, and interleukins. -
Q: Can you customize an assay to meet my project’s specific needs?
A: Yes. Many of our clients require assay adaptations for unique sample types or research goals. We can tailor protocols, adjust assay conditions, or validate for factors such as cross-reactivity and sample extraction. This ensures the results are relevant and reliable for your particular study. -
Q: What does your pricing include?
A: Our pricing is all-inclusive, covering assay reagents, sample preparation, labor, data generation, and a final report in an easy-to-interpret format. Additional costs may apply if specialized validation or extended purification is needed, but all details are discussed transparently upfront. -
Q: How do you ensure data quality and reproducibility?
A: All assays are performed under strict quality control measures by experienced scientists. We use validated protocols and high-quality reagents to minimize variability. Every dataset is carefully analyzed and reviewed to ensure accuracy and consistency across replicates. -
Q: How do I submit samples, and what matrices can you work with?
A: We accept a wide range of biological matrices, including serum, plasma, tissue extracts, and cell culture supernatants. Our team will provide detailed guidelines for sample preparation and shipping to ensure integrity upon arrival. -
Q: How quickly can I expect results?
A: Turnaround times vary depending on the assay and sample complexity, but most projects are completed within 2–4 weeks from sample receipt. For urgent timelines, we can often expedite services upon request.
Resources
Related Services
- Protein Analytical Service
- Custom Protein Service
- Biopharmaceutical Solution
- Experiment Consulting and Design Service
- Cytokine and Receptor Analysis
- Enzyme Activity Assay
- Protein Kinase Assay
Related Products
References:
- Foudah AI, Alqarni MH, Alam A, Salkini MA, Ross SA, Yusufoglu HS. Phytochemical screening, in vitro and in silico studies of volatile compounds from Petroselinum crispum (Mill) leaves grown in Saudi Arabia. Molecules. 2022;27(3):934. doi:10.3390/molecules27030934
- Hawerkamp HC, Dyer AH, Patil ND, et al. Characterisation of the pro-inflammatory cytokine signature in severe COVID-19. Front Immunol. 2023;14:1170012. doi:10.3389/fimmu.2023.1170012
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

