Multimode Chromatography
Background
Overview
Multimodal Chromatography is a chromatographic analysis technique that combines two or more chromatographic modes to improve the efficiency and selectivity of the analysis. This technique allows for more efficient separation and identification of various components in complex samples by using different columns or chromatographic conditions in the same chromatographic system. Such as liquid-solid adsorption chromatography, liquid-liquid partition chromatography (positive phase and reverse), ion exchange chromatography, ion pair chromatography and molecular exclusion chromatography.
The core of multi-mode chromatography is to use the characteristics of different chromatographic modes to achieve better separation results. By combining these chromatographic modes, it is possible to achieve efficient separation and analysis of complex samples. Chromatographic modes mainly include the following:
Gas chromatography (GC). Suitable for volatile and thermally stable samples, the sample is separated in the column based on the interaction with the stationary phase by using the gas as the mobile phase.
Liquid chromatography (LC). Suitable for non-volatile or thermally unstable samples, using liquid as the mobile phase and sample separation based on its allocation between the stationary and mobile phases.
Thin layer chromatography (TLC). A simple chromatography technique for rapid separation and qualitative analysis.
Gel permeation chromatography (GPC). Used to separate polymers or macromolecular compounds with different molecular weights.
Ion exchange chromatography (IEC). Separation using interactions between ions in a sample and ion-exchange groups in a stationary phase.
Affinity chromatography. Based on specific interactions between biomolecules, such as antigen-antibody, enzyme-substrate, etc.
Advantages of Multimodal Chromatography
High resolution: By combining different chromatographic modes, the separation resolution of complex samples can be significantly improved.
High selectivity: Different chromatographic modes can be optimized for specific compounds or classes of compounds, increasing the selectivity of the analysis.
Flexibility: Multi-mode chromatography can flexibly select and combine chromatographic modes according to the characteristics of the sample and the purpose of analysis.
Rich in information: Combining multiple chromatographic modes allows more sample information to be obtained, contributing to a fuller understanding of the composition and properties of the sample.
Technical Challenges
Interface devices and technologies. In multi-mode chromatography, especially two-dimensional chromatography, the key is to connect two different selective separation columns or different types of chromatographic separation systems. This requires efficient and stable interface equipment and technology to ensure efficient sample transfer between the two separation systems while maintaining the separation effect.
Stability of the sample. Especially in the purification process of biological macromolecules such as mRNA, due to their large molecular size, relatively single chemical composition and instability, it is difficult to purify by chromatography. The high similarity of the by-product to the target molecule also increases the difficulty of purification.
The increase in complexity. In the field of biopharmaceuticals, especially when analyzing increasingly complex biopharmaceutical materials, such as monoclonal antibodies and antibody-coupled drugs, the safety and efficacy of these protein molecules are obvious, but their complexity also brings new challenges to the analysis and application of multimodal chromatography.

Consideration Factors
In principle, the composition of multimodal chromatography requires more than two chromatographic modes. When selecting multimodal chromatography, it is necessary to consider sample characteristics, separation objectives, chromatographic conditions, column selection, economic and time costs, and the specific requirements of the application field. Some of these important factors are discussed below:
Chemical properties of the sample
Polarity. The polarity of the sample molecules will affect their retention behavior in different chromatographic modes. Polar molecules may be better suited to use hydrophilic interaction chromatography (HILIC) or ion exchange chromatography (IEC).
Size and shape. Large molecules such as proteins may require volume exclusion chromatography (SEC) for separation, while small molecules may be better suited using reverse-phase chromatography (RPC).
Electric charge. Charged sample molecules may need to be separated using IEC or affinity chromatography based on charge interactions.
Hydrophobic. Hydrophobic molecules have better retention in RPC and may be less retention in HILIC.
Separate target
The resolution. It is necessary to consider whether the goal is simple separation or high resolution analysis of complex mixtures. Multimodal chromatography can provide higher resolution and help solve the separation problem of complex samples.
Be selective. Selectivity refers to the ability of chromatography to distinguish between adjacent compounds. Multimodal chromatography can improve selectivity by combining different separation mechanisms.
Chromatographic condition
The mobile phase. The composition of mobile phase (such as buffer pH, organic solvent ratio) has significant influence on the separation effect. The mobile phase needs to be adjusted according to the sample nature and chromatographic mode.
The temperature. Temperature control is essential to maintain the stability and reproducibility of the chromatographic process.
Applications
Multimodal Chromatography, also known as mixed mode chromatography, is a highly efficient chromatographic technique that combines two or more chromatographic separation mechanisms, such as ion exchange, affinity, size exclusion, and hydrophilic interaction chromatography (HIC). The advantage of this method is that it can improve the selectivity and peak capacity of protein purification, while reducing the number of chromatographic steps required in the purification process. Here are some of the main application areas of multimodal chromatography:
- Biopharmaceutical downstream processing. In the field of biopharmaceuticals, multimodal chromatography is an important tool for protein purification. Due to increasingly stringent regulatory requirements for protein purity, the need to develop improved chromatographic resins for chromatographic separation is increasing. Multimodal chromatography can improve the specificity of the interaction between proteins and ligands on the chromatographic medium by combining different chromatographic mechanisms, thus improving the purification efficiency and selectivity.
- Complex sample analysis. Multimodal chromatography is particularly suitable for the analysis of complex samples such as biological, environmental and food samples. It can isolate and detect hundreds of components and their metabolites and products of transition forms in a single analytical process. By combining different chromatographic modes, multimodal chromatography can improve the resolution and peak capacity of the analysis and help to solve the separation problem of complex samples.
- Proteomic studies. In proteomics research, multimodal chromatography can be used to identify and quantify proteins. It can effectively isolate and purify proteins, especially those that are difficult to separate by a single chromatographic mode. In addition, multimodal chromatography can also be used to study the structure/function relationship of proteins.
- Drug analysis. In drug development and quality control, multimodal chromatography can be used for the analysis, purification and structural identification of drug components. It is particularly useful for analyzing active ingredients, metabolites, and impurities in drugs.

Case Study
Case Study 1: Anion Exchang MMC
In this work, a mechanistic modeling aided approach were present that paves the way for an accelerated development of anionic mixed-mode chromatography (MMC) for biopharmaceutical purification. A modified multimodal isotherm model was calibrated using only three chromatographic experiments and was employed in the retention prediction of four antibody formats including a Fab, a bispecific, as well as an IgG1 and IgG4 antibody subtype at pH 5.0 and 6.0. An existing multimodal isotherm model was reduced to hydrophobic interactions in the linear range of the adsorption isotherm and successfully employed in the simulation of six chromatographic experiments per molecule in concert with the transport dispersive model (TDM). The model reduction to only three parameters did prevent structural parameter non-identifiability and enabled an analytical isotherm parameter determination that was further refined by incorporation of size exclusion effects of the selected multimodal resin. During the model calibration, three linear salt gradient elution experiments were performed for each molecule followed by an isotherm parameter uncertainty assessment. Lastly, each model was validated with a set of step and isocratic elution experiments.
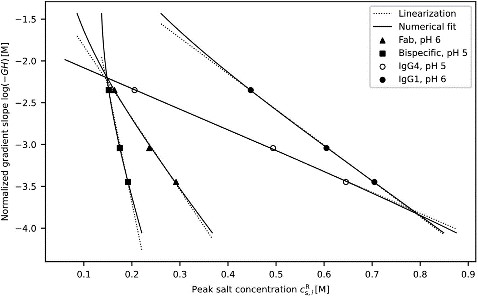
(Rudger Hess, 2023)
Fig1. Analytical and numerical isotherm parameter estimation shown for four different antibody formats at pH 5.0 and 6.0.
Case Study 2: Cation Exchanger MMC
A novel salt-tolerant cation-exchange membrane, prepared with a multimodal ligand, 2-mercaptopyridine-3-carboxylic acid (MMC-MPCA), was examined for its purification properties in a bind-and-elute mode from the high conductivity supernatant of a Pichia pastoris fermentation producing and secreting a single-chain variable fragment (scFv). Two fed-batch fermentations of Pichia pastoris resulted in fermentation supernatants reaching an scFv titer of 395.0 mg/L and 555.7 mg/L, both with a purity of approximately 83 %. The MMC-MPCA membrane performance was characterized in terms of pH, residence time (RT), scFv load, and scFv concentration to identify the resulting dynamic binding capacity (DBC), yield, and purity achieved under optimal conditions. The MMC-MPCA membrane exhibited the highest DBC of 39.06 mg/mL at pH 5.5, with a residence time of 1 min, while reducing the pH below 5.0 resulted in a significant decrease of the DBC to around 2.5 mg/mL. With almost no diffusional limitations, reducing the RT from 2 to 0.2 min did not negatively impact the DBC of the MMC-MPCA membrane, resulting in a significant improvement in productivity of up to 180 mg/mL/min at 0.2 min RT. When performing the purification under the optimized conditions, the resulting purity of the product improved from 83 % to approximately 92-95 %.
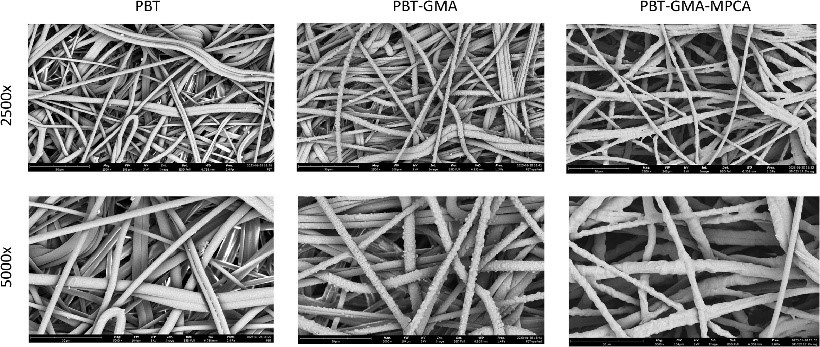
(Dan N Pham, 2024)
Fig2. Scanning electron microscopy images of PBT, PBT-GMA and PBT-GMA-MPCA (MMC-MPCA) membranes with 2500x and 5000x magnification.
Case Study 3: Mixed-Mode Resin
The mass-transfer process of l-tryptophan (l-Trp) in the hydrophobic interaction/ion-exchange mixed-mode resin HD-1 particles and fixed bed was studied experimentally and theoretically. The adsorption kinetics of l-Trp in single-component and multicomponent adsorption systems was investigated under different pH conditions. A modified liquid-film linear driving force model considering the physical adsorption of l-Trp zwitterions and anions as well as ion exchange of l-Trp cations was proposed. The dissociation equilibria of l-Trp molecules and functional groups on the resin were introduced in the model. The presence of Na+ and the impurity amino acid l-glutamic acid (l-Glu) did not significantly affect the mass-transfer rate of l-Trp. The dynamic adsorption processes of l-Trp under different pH and concentration conditions were studied. During the dynamic adsorption process, the pH of mobile phase in the fixed bed changed with changing the l-Trp concentration in the mobile phase. l-Trp was well separated from Na+ and l-Glu with the purity of l-Trp higher than 99%, the recovery rate higher than 95%, and a concentration of 4.69 × 10-3 mol/L. The elution chromatographic peaks of l-Trp, l-Glu, and Na+ and the pH of the outlet solution were predicted satisfactorily.
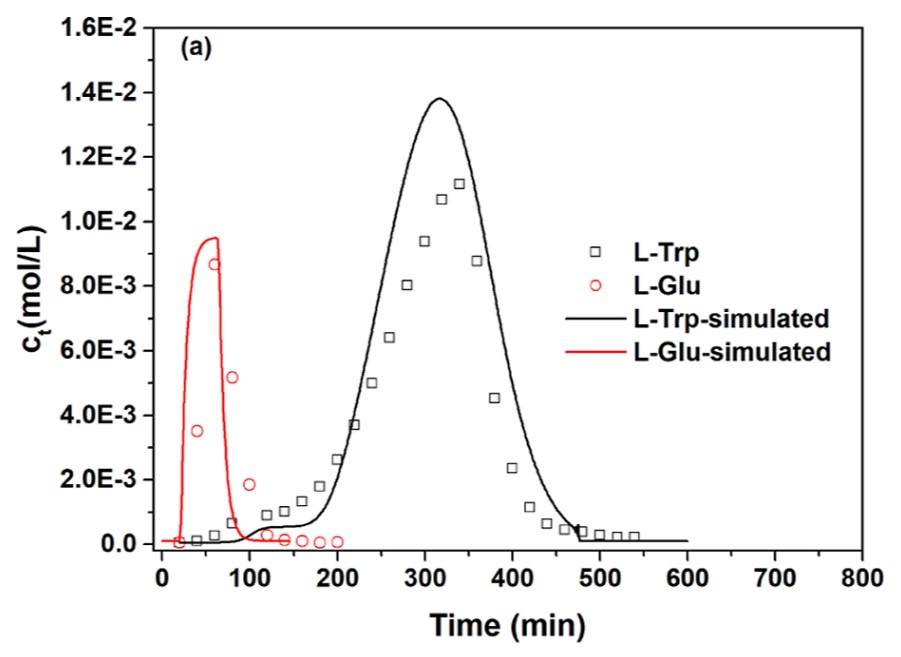
(Pengfei Jiao, 2022)
Fig3. Chromatographic peaks of l-Trp and l-Glu.
Advantages
- Customized services. We can provide customized affinity fillers and services to meet the requirements of different biological processes according to the specific needs of customers.
- Wide range of products. We are able to provide a wide range of products related to multi-mode chromatography, with a rich product line to meet the needs of different users.
- Market recognition. Because of the high quality of our products, we have a certain reputation and recognition in the market, you can rest assured to buy our products.
Creative BioMart can provide various types of combination products to meet customer’s different research goals. You can also let us know if you have any customized requirements. Please contact us for more product details.
References
- Hess R.; et al. Standardized method for mechanistic modeling of multimodal anion exchange chromatography in flow through operation. J Chromatogr A. 2023;1690:463789.
- Pham DN.; et al. Novel multimodal cation-exchange membrane for the purification of a single-chain variable fragment from Pichia pastoris supernatant. J Chromatogr A. 2024;1718:464682.
- Jiao P.; et al. Simulation of Adsorption Process of l-Tryptophan on Mixed-Mode Resin HD-1 with Combined Physical Adsorption and Ion Exchange. ACS Omega. 2022;7(39):35331-35338.










