Protease Assay Service
Background
The human genome encodes nearly 500 putative proteases, each playing vital roles in physiological regulation through proteolytic activity. Proteases such as DPP-IV and HIV-1 protease have already been successfully targeted for the treatment of diabetes and AIDS. However, the functions of many proteases remain poorly understood—especially in the context of complex diseases.
To accelerate drug discovery involving protease targets, Creative BioMart offers a comprehensive Protease Assay panel for high-throughput screening and profiling. For more specialized needs, we also provide custom assay development tailored to your target of interest.
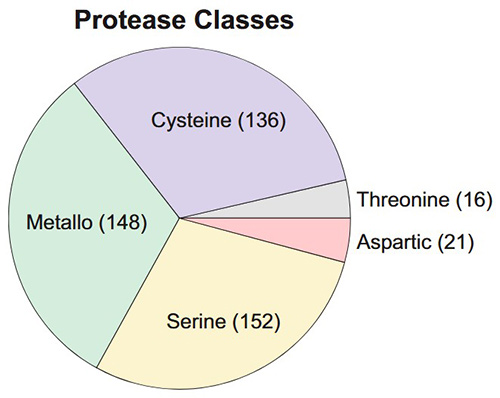
Figure 1. Types of protease classes. (De Almeida et al., 2022)
What We Offer?
Service Procedure

- Sample Preparation: Prepare and dilute test samples appropriately to ensure optimal assay performance and reproducibility.
- Incubation with Labeled Substrate: Incubate the samples with a specific fluorogenic or chromogenic protease substrate under controlled conditions.
- Enzymatic Reaction: Allow the protease, if present, to cleave the substrate, generating a measurable signal (e.g., fluorescence or absorbance).
- Signal Detection: Measure fluorescence (or absorbance) using a microplate reader to detect proteolytic activity.
- Data Analysis: Identify positive samples exhibiting significant signal changes indicative of protease activity.
- Quantification (Optional): Determine protease concentration by comparing sample signal to a standard curve generated from known protease standards.
- Reporting & Interpretation: Deliver a comprehensive report including raw data, activity profiles, and optional recommendations for follow-up experiments or screening.
Service Highlights
| Types | Details |
|---|---|
|
Activity Assays |
|
|
Inhibition Assays |
|
|
Zymography |
|
|
Protease Profiling |
|
Why Choose Us?
- Broad Protease Coverage: Our assays support a wide range of proteases, including serine, cysteine, metalloproteases, and aspartic proteases, for diverse research applications.
- Flexible Project Design: We offer fully customizable workflows—from enzyme sources and substrate types to readouts—to match your scientific and regulatory goals.
- Multiple Detection Formats: Choose from fluorescence, luminescence, FRET, or colorimetric readouts, all optimized for sensitivity, throughput, and kinetic profiling.
- High Sensitivity & Specificity: Assays are rigorously validated to ensure reliable detection of subtle activity changes and accurate inhibitor profiling in complex samples.
- Mechanism of Action Analysis: We provide detailed kinetic parameters, including IC50, Ki, and mode-of-inhibition, to support hit validation and lead optimization efforts.
- Expert Scientific Support: Our team assists with assay selection, data interpretation, and troubleshooting to ensure your protease study delivers actionable insights.
Case Study
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Collagen derived Gly-Pro-type DPP-IV inhibitory peptides
Xu et al., 2024. doi:10.1016/j.foodchem.2024.138370
This study investigated how amino acid composition and peptide length influence the DPP-IV inhibitory activity of Gly-Pro-type peptides. Using QSAR modeling, enzymatic kinetics, and molecular docking, researchers found that lower electrophilicity, polarity, and side-chain bulk at the N3 position enhance inhibition. Longer peptides (3–12 residues) maintained competitive inhibition but showed improved binding and lower Ki values. Peptides with 5–6 residues interacted more extensively with DPP-IV subsites, enhancing inhibition by stabilizing key binding interactions and reducing acylation.
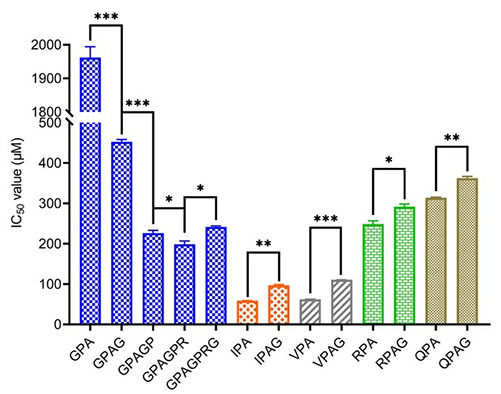
Figure 2. Dipeptidyl-peptidase IV (DPP-IV) half-maximal inhibitory concentration (IC50) of XPA-type peptides with varied lengths. (Xu et al., 2024)
Case 2: Characterization of an industrially viable aspartic protease from Aspergillus niger
Purushothaman et al., 2019. doi:10.1016/j.ijbiomac.2019.07.133
An aspergillopepsin A-like aspartic protease from Aspergillus niger was purified and characterized following solid-state fermentation. The 50 kDa enzyme exhibited optimal activity at pH 3.5 and 60 °C, remaining stable for 60 minutes at 50 °C. It showed high specific activity (40,000 U/mg) and 85% sequence homology with known Aspergillus proteases. Inhibition by pepstatin A (Ki = 0.045 μM) and β-rich secondary structure were confirmed. The enzyme effectively hydrolyzed various substrates and improved defatted soya flour functionality, making it suitable for food and feed applications.
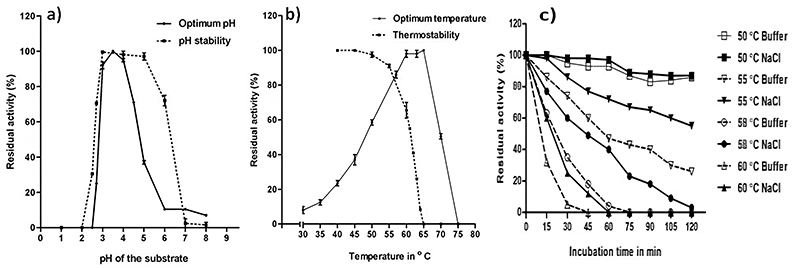
Figure 3. Kinetic parameters of purified aspartic protease. (Purushothaman et al., 2019)
Customer Testimonials
-
“We used Creative BioMart’s MMP-9 fluorescence assay to profile our inhibitor panel, and the results were spot-on. The turnaround was fast, and the team provided detailed kinetic analysis that helped us prioritize leads for in vivo testing.”
— Senior Scientist | Biotech Startup (Oncology Focus)
-
“Creative BioMart’s customized DPP-IV inhibition assay helped us screen a library of over 200 compounds efficiently. We especially appreciated the consistent IC50 values across multiple runs—truly reliable data.”
— R&D Director | Mid-Sized Pharmaceutical Company
-
“We collaborated on a cathepsin B assay for a lysosomal dysfunction study. The assay was sensitive enough to detect subtle activity shifts in tissue lysates. Their scientific support was top-notch.”
— Principal Investigator | Academic Research Institute
-
“We needed a high-throughput, FRET-based protease panel covering several cysteine proteases. Creative BioMart delivered a scalable solution, along with full data analysis that saved us a ton of time.”
— Drug Discovery Manager | Global CRO
-
“Our project on calpain inhibitors had very specific requirements. Creative BioMart’s customized assay conditions precisely, and the resulting data helped secure our next round of funding.”
— Lead Scientist | Rare Disease Foundation Lab
FAQs
-
Q: What types of proteases can you assay?
A: We cover a wide range of proteases, including serine, cysteine, aspartic, threonine, and metalloproteases. Whether it’s MMPs, caspases, cathepsins, or DPPs, we can design assays to match your target class and research needs. -
Q: Can you customize assays for my specific inhibitor or substrate?
A: Absolutely. We tailor assay conditions—including pH, buffer composition, substrates, and detection methods—to align with your compound properties and biological context. -
Q: What detection formats do you offer?
A: Our platform supports fluorescence, luminescence, FRET, and colorimetric readouts. We choose the best format based on your assay goals, throughput, and sensitivity requirements. -
Q: How do you ensure data quality and reproducibility?
A: All assays undergo rigorous optimization and QC validation. We use internal standards, positive/negative controls, and replicate testing to ensure consistent, high-quality results. -
Q: Do you offer kinetic analysis or just endpoint readouts?
A: Both are available. We provide full kinetic data (Km, Vmax, IC50, Ki) and mechanistic insights to help you understand inhibitor potency and mode of action.
-
Q: What is your typical turnaround time?
A: Most standard protease assays are completed within 2–3 weeks. Timelines for customized or high-throughput projects may vary based on complexity and scale.
Resources
Related Services
Related Products
References:
- De Almeida LGN, Thode H, Eslambolchi Y, et al. Matrix metalloproteinases: from molecular mechanisms to physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 2022;74(3):714-770. doi:10.1124/pharmrev.121.000349
- Purushothaman K, Bhat SK, Singh SA, Marathe GK, Appu Rao ARG. Aspartic protease from Aspergillus niger: Molecular characterization and interaction with pepstatin A. International Journal of Biological Macromolecules. 2019;139:199-212. doi:10.1016/j.ijbiomac.2019.07.133
- Xu Q, Zheng L, Huang M, Zhao M. Collagen derived Gly-Pro-type DPP-IV inhibitory peptides: Structure-activity relationship, inhibition kinetics and inhibition mechanism. Food Chemistry. 2024;441:138370. doi:10.1016/j.foodchem.2024.138370
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

