Chemokine Signaling Pathway
🧪 KRAS-2569H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-185 aa

🧪 EIF4E-2775H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-217 aa


🧪 CREB1-26H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 2-328 a.a.
🧪 AKT3-131H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-479 a.a.
🧪 JUN-1027H
Source: Insect Cells
Species: Human
Tag: Non
Conjugation:
Protein Length: 1-331 aa
🧪 RAF1-1123H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 50-132 a.a.
🧪 RHOA-1145H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:

🧪 STAT3-1496H
Source: Sf9 Cells
Species: Human
Tag: GST
Conjugation:
Protein Length:

🧪 STAT5A-1498H
Source: Sf9 Cells
Species: Human
Tag: His
Conjugation:
Protein Length:
🧪 GSK3B-616H
Source: Insect Cells
Species: Human
Tag: His
Conjugation:
Protein Length: 1-433 aa

🧪 RAC1-2213H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: Met1-Cys189
🧪 AKT1-786H
Source: Insect Cells
Species: Human
Tag: His
Conjugation:
Protein Length: 1-480 aa
🧪 GRK5-760H
Source: Insect Cells
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Ser590
🧪 MAPK8-781H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: Met1-Arg427
🧪 STAT1-2783H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-712 a.a.
Background of Chemokine Signaling Pathway
What is Chemokine?
Chemokines are small proteins, usually ~70–80 amino acid residues, with conserved sequence and structural features and expressed in tissues during normal immune surveillance or in response to injury or infection. Chemokines are designated according to their subfamily classification by systematic names composed of a prefix (CCL, CXCL, CX3CL, or XCL; “L” signifies a ligand as opposed to a receptor) followed by an identifying number.
Chemokines are defined by their primary amino acid sequence and the arrangement of specific structurally important cysteine residues within the mature protein. Variation in the precise configuration of the two cysteines closest to the N terminus allows chemokines to be split into four subfamilies: CC, CXC, CX3C, and XC. In CC chemokines, these cysteines are directly juxtaposed, while CXC chemokines have a single variable amino acid between them. The sole CX3C chemokine has three amino acids between these two cysteines, while XC chemokines, of which there are two forms in humans and one in mice, lack the first and the third cysteines of the motif.
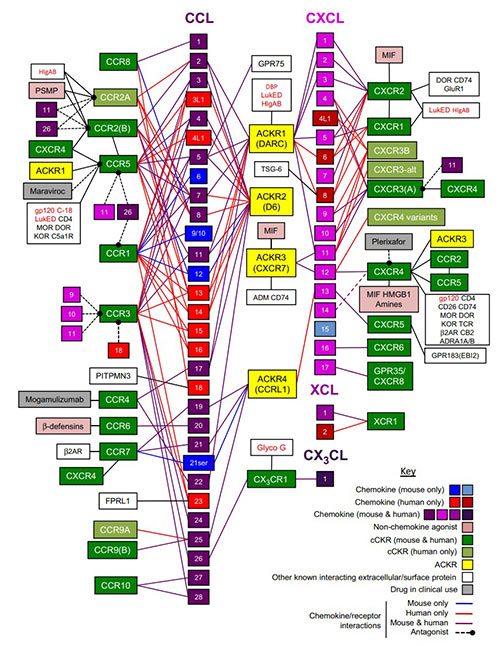
Fig1. Mammalian chemokine receptors and their known interactions with chemokines and other key secreted, cell surface, and pathogen‐encoded molecules.
What is Chemokine Receptor?
Chemokines bind and activate chemokine receptors, G protein-coupled receptors (GPCRs) embedded in the cell membranes of leukocytes, thereby inducing leukocyte adhesion to the vessel wall, morphological changes, extravasation into the inflamed tissue, and chemotaxis along the chemokine gradient to the site of injury or infection.
Mammalian genomes each encode approximately 20 chemokine receptors. Because the receptors were discovered after the chemokines and most of them are selective for members of one chemokine subfamily, they are classified according to the subfamily of chemokines to which most of their ligands belong. Thus, receptors are named using the prefixes CCR, CXCR, CX3CR, and XCR followed by an identifying number.
Different subsets of leukocytes express different arrays of chemokine receptors, enabling them to respond to the appropriate ligands. Most chemokines bind and activate several receptors. Similarly, most chemokine receptors respond to multiple chemokine ligands. This selectivity of recognition is an intrinsic property of the chemokine-receptor pair. However, selectivity can be altered by modification of the proteins.
Typically, chemokine receptor stimulation leads to the GDP/GTP exchange of coupled heterotrimeric Gi proteins and the subsequent dissociation of the βγ subunits, which then activate phosphoinositide-specific phospholipase Cβ (PLC) and phosphoinositide 3-kinase (PI3K). PLC produces inositol-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers calcium mobilization whereas DAG activates protein kinase C (PKC). PI3K generates 3-phosphoinositides, which serve as anchors in the recruitment of proteins with pleckstrin homology domains to the plasma membrane, such as AKT/PKB. Although these signaling events are common to all chemokine receptors, it is well known that the activation of further downstream pathways is quite different. This may depend on the efficacy with which a chemokine triggers its receptor, giving rise to different spatial and temporal signal fluxes.
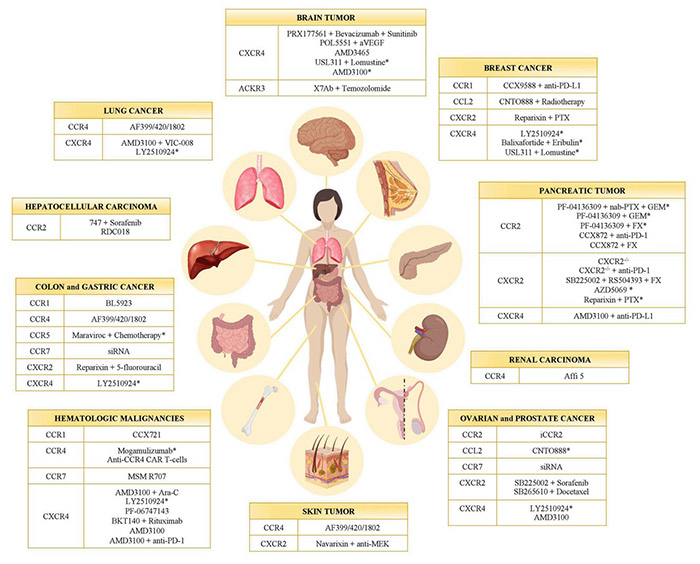
Fig2. Chemokine receptor inhibitors in cancer. (Valeria Mollica Poeta, 2019)
What is Chemokine Signaling Pathway?
Upon chemokine binding, the associated G protein is activated, leading to the dissociation of its subunits and the activation of various downstream pathways. These include the JAK/STAT pathway, the phospholipase C (PLC) pathway, and the PI3K/Akt pathway.
The activation of these pathways results in cellular responses such as cytoskeletal rearrangements, cell survival, proliferation, and migration. These are essential for processes like immune cell trafficking to sites of infection or inflammation, and for the development and organization of tissues.
Dysregulation of chemokine signaling can contribute to chronic inflammatory diseases, autoimmune disorders, and cancer. High levels of chemokines or unregulated signaling can lead to persistent inflammation, while in cancer, chemokines can influence tumor growth and metastasis.
Case Study
Case Study 1: Recombinant Human CCL19 protein (CCL19-721H)
Short linear peptides can overcome certain limitations of small molecules for targeting protein-protein interactions (PPIs). Herein, the interaction between the human chemokine CCL19 with chemokine receptor CCR7 was investigated to obtain receptor-derived CCL19-binding peptides. After identifying a linear binding site of CCR7, five hexapeptides binding to CCL19 in the low micromolar to nanomolar range were designed, guided by pharmacophore and lipophilicity screening of computationally generated peptide libraries. The results corroborate the applicability of the computational approach and the chosen selection criteria to obtain short linear peptides mimicking a protein-protein interaction site.
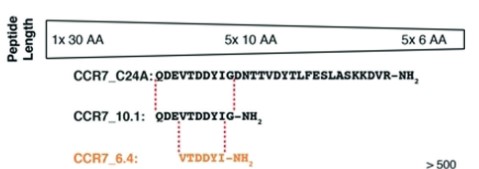
Fig1. CCR7_C24A was cutinto five overlapping10-mers. (Jens-Alexander Fuchs, 2019)
Case Study 2: Recombinant Rat Chemokine (C-C Motif) Ligand 5 (Ccl5-104R)
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of immature cells that are believed to inhibit immune responses in the contexts of cancer and organ transplantation, in association with regulatory T cells (Treg). However, the way in which MDSC cooperate with Treg remains elusive. In this study, the researchers used DNA microarrays to analyze gene expression in blood-derived MDSC from rat recipients of kidney allografts. They found CCL5 (Rantes), a chemotactic C-C motif 5 chemokine, to be strongly downregulated after treatment with a tolerizing regimen. The amount of CCL5 protein was also lower in the plasma of tolerant recipients, whereas intragraft CCL5 was unchanged. Because CCL5 is chemotactic for Treg, they hypothesized that a gradient of CCL5 between the graft and peripheral blood might contribute to the intragraft localization of Treg in tolerant animals. To test this hypothesis, they treated tolerant rat recipients of kidney allografts with recombinant rat CCL5 to restore normal plasma concentrations. This led to a strong reduction in intragraft Treg monitored by immunohistofluorescence and by quantitative real-time PCR measurement of Foxp3 mRNA.
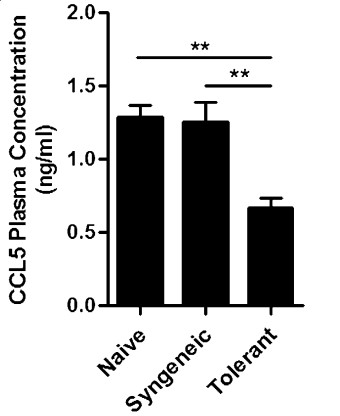
Fig2. Quantification of CCL5 protein levels by ELISA in plasma of nongrafted rats. (Nahzli Dilek, 2012)
Case Study 3: Recombinant Human Ras Homolog Gene Family (RHOA-3372H)
Traditional glycosyltransferase (GT) activity assays are not easily configured for rapid detection nor for high throughput screening because they rely on radioactive product isolation, the use of heterogeneous immunoassays or mass spectrometry. In a typical glycosyltransferase biochemical reaction, two products are generated, a glycosylated product and a nucleotide released from the sugar donor substrate. Therefore, an assay that detects the nucleotide could be universal to monitor the activity of diverse glycosyltransferases in vitro. Here the researchers describe three homogeneous and bioluminescent glycosyltransferase activity assays based on UDP, GDP, CMP, and UMP detection. Each of these assays are performed in a one-step detection that relies on converting the nucleotide product to ATP, then to bioluminescence using firefly luciferase. These assays are highly sensitive, robust and resistant to chemical interference. Various applications of these assays are presented, including studies on the specificity of sugar transfer by diverse GTs and the characterization of acceptor substrate-dependent and independent nucleotide-sugar hydrolysis.
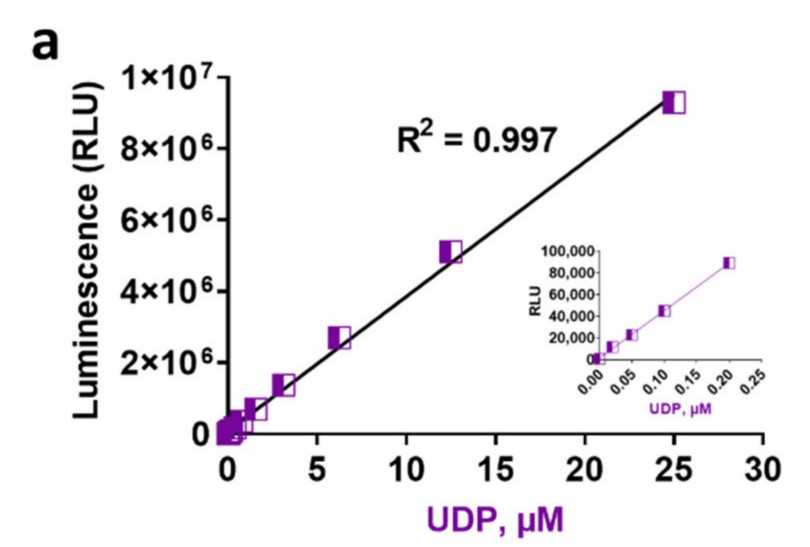
Fig3. Linearity and sensitivity of bioluminescent nucleotide assays. (Laurie Engel, 2021)
Related Products
By far the most studied function of the chemokine network is cell migration, particularly of leukocytes. In addition to their roles in leukocyte trafficking, chemokine activation of chemokine receptors can give rise to a variety of additional cellular and tissue responses, including proliferation, activation, differentiation, extracellular matrix remodeling, angiogenesis, and tumor metastasis. Creative BioMart can provide a list of core protein products of the chemokines and chemokine receptors to help you with your researches. Please feel free to contact us if you’re interested.


