Radioimmunoassay
Background
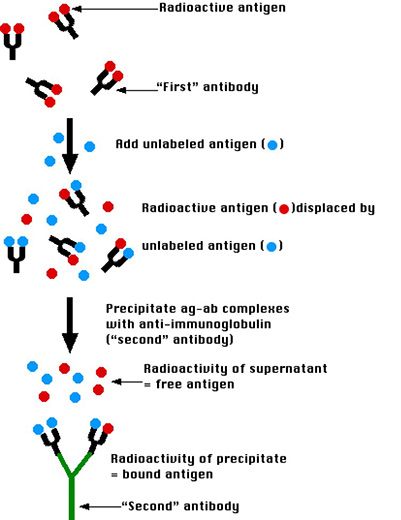
Radioimmunoassay (RIA) is a highly sensitive and specific technique that combines the precision of radioactive detection with the specificity of antibodies to quantify trace amounts of antigens in biological samples. First developed in the 1960s, RIA remains a gold standard for hormone, drug, and protein quantification due to its unparalleled precision.
Principle
The fundamental principle of RIA is competitive binding. A known quantity of radioactively labeled antigen (the tracer) competes with the unlabeled antigen in the sample for a limited number of high-affinity antibody binding sites. As the concentration of the unlabeled antigen increases, it displaces more of the tracer, reducing the radioactivity bound to the antibody complex. This inverse relationship allows for the accurate determination of antigen concentrations as low as a few picograms per milliliter, depending on antibody affinity (Kd = 10-8 – 10-11 M).
Commonly used isotopes include Iodine-125 (125I), and occasionally Carbon-14 (14C) and Tritium (3H). The radioactive antigens are typically prepared by iodinating the antigen on its tyrosine residues using chloramine-T or peroxidase methods, followed by purification through gel filtration or HPLC.
Applications of Radioimmunoassay
RIA is extensively used in both clinical and research settings. Common applications include:
- Endocrinology: Measuring insulin, thyroid hormones, cortisol, and growth hormone levels
- Oncology: Early cancer biomarker detection (e.g., PSA, CEA)
- Neurology: Quantification of neurotransmitters and neuropeptides
- Immunology: Detection of cytokines and viral antigens
- Toxicology: Detection of drugs and narcotics in biological samples
- Gastroenterology: Diagnosing and monitoring treatment for peptic ulcers via gastrin measurements
- Virology: Screening for hepatitis and tracking of leukemia virus presence
At Creative BioMart, we offer advanced Radioimmunoassay Services tailored to meet the demands of academic research, clinical studies, and pharmaceutical development. Leveraging high-specificity radiolabeled antibodies, our assays deliver precise quantitation even at picogram levels—ideal for biomarker validation, pharmacokinetics, endocrinology, and immunology applications. With rigorous quality control, customizable assay formats, and rapid turnaround, our RIA platform ensures reproducible data with superior sensitivity and reliability.
What We Offer?
Service Procedure

Our platform is designed to provide detailed insights into the antigenic regions that are essential for antibody binding, supporting rational design and downstream validation. Whether your goal is to characterize monoclonal antibodies, evaluate antigenic sites, or confirm binding domains, our tailored solutions are optimized for performance and accuracy.
Service Details
- Analyte range: Hormones, peptides, small molecules, neurotransmitters
- Sample types: Serum, plasma, urine, tissue homogenates, cell culture supernatants
- Isotopes used: I-125 (primary), H-3 and C-14 (upon request)
- Detection limits: Down to picogram levels
- Turnaround time: Typically, 1–3 weeks, depending on project complexity
- Customization: Fully customizable assays tailored to your experimental requirements
Why Choose Us?
- Proven Expertise: Decades of experience in immunoassay development and execution, with a specialized team dedicated to radiolabeling techniques.
- Custom Solutions: Each RIA is uniquely tailored to your research or diagnostic needs, ensuring relevance and precision.
- High Sensitivity & Specificity: We use high-affinity antibodies and high-specific-activity tracers for optimal assay performance.
- Regulatory Compliance: All radioactive materials are handled in accordance with safety and regulatory guidelines.
- Strict Quality Control: All assays undergo thorough validation and are run in triplicate to ensure reproducibility.
- Global Reach: Serving clients across academia, biotech, pharma, and healthcare industries worldwide.
Case Study
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Radioimmunoassay for human angiotensin-(1-12) measurement in plasma and urine
Ahmad et al., 2021. doi:10.1016/j.mce.2021.111256
Angiotensin-(1–12) [Ang-(1–12)] is a dodecapeptide that serves as an intracrine and paracrine precursor for local angiotensin II production. In this study, a highly specific and reliable radioimmunoassay (RIA) was developed to quantify Ang-(1–12) levels in human plasma and urine using an affinity-purified polyclonal antibody targeting the C-terminal region of the peptide. The assay was applied to samples from thirty-four individuals, including twenty-nine patients receiving antihypertensive therapy and five untreated subjects. Plasma Ang-(1–12) concentrations were found to be significantly elevated in individuals with systolic blood pressure ≥140 mm Hg compared to those with lower systolic pressure, while no significant difference was observed in urinary Ang-(1–12) levels between these groups.
Figure 1. Linearity assay of pooled plasma (A) and pooled spot urine (B). (Ahmad et al., 2021)
Case 2: Radioimmunoassay for estimation of C‑peptide in human serum
Rasmi et al., 2021. doi:10.1007/s10967-020-07536-4
The C-peptide radioimmunoassay (RIA) has proven to be a powerful tool in diagnosing conditions like diabetes, insulinomas, and various forms of hypoglycemia. This particular RIA was developed using a polyclonal antibody raised in rabbits, specifically targeting human C-peptide, and its performance was validated with human serum samples. To separate the antibody-bound complexes from the free ones, a polyethylene glycol solution was used. Both within-run and between-run consistency showed strong reliability, and recovery rates fell well within acceptable analytical standards. Importantly, the assay showed no cross-reactivity with other similar peptides and hormones, such as proinsulin, glucagon, somatostatin, insulin, or pancreatic polypeptide.
Figure 2. Cross reactivity of C-peptide RIA with related protein hormones. (Rasmi et al., 2021.)
Customer Testimonials
-
"Our lab needed a highly sensitive glucagon RIA to track subtle hormonal fluctuations in our metabolic study cohorts. The turnaround time was fast, the support was incredibly responsive, and the assay's reproducibility across runs saved us from repeating costly experiments. Top-tier service all around."
— Senior Scientist | Academic Medical Research Institute
-
"We’ve been routinely using Creative BioMart's RIA services for patient sample analysis, particularly for measuring pancreatic polypeptide and C-peptide levels. The consistent results across batches and the minimal inter-assay variability have made these services a staple in our diagnostic workflow. It’s rare to find high throughput and high accuracy in one service."
— Clinical Lab Supervisor | University Hospital
-
"We were tackling a project involving somatostatin regulation and needed reliable quantification at low concentration ranges. The Creative BioMart’s radioimmunoassay service delivered excellent detection limits and negligible cross-reactivity. It gave us the resolution we needed without complicating the workflow."
— Principal Investigator | Government Research Lab
-
"We had a hard-to-detect neurotransmitter and Creative BioMart nailed it with their custom RIA. Would absolutely recommend them for sensitive assays."
— Principal Investigator | Neurobiology Lab
FAQs
-
Q: What sample volume is required for RIA?
A: Typically, 100–200 µL of serum or plasma per replicate. For low-abundance analytes, higher volumes may be needed. -
Q: Is RIA safe given the use of radioactivity?
A: Yes. Our facilities adhere to strict radiological safety protocols and handle all isotopes in controlled environments. -
Q: Can I request a custom antigen or antibody for the assay?
A: Absolutely. We offer fully customizable RIA setups, including custom antibody production and tracer synthesis. -
Q: How does RIA compare to ELISA?
A: RIA offers higher sensitivity, particularly for very low-abundance analytes, although ELISA is more commonly used due to the absence of radioactivity. -
Q: Do you provide regulatory documentation?
A: Yes, we provide detailed assay protocols, validation data, and compliance documentation upon request.
Resources
Related Services
- Direct Peptide Reactivity Assay (DPRA)
- Metabolism Assays
- High-Throughput Screening
- Quality Control
- Bioanalytical Assay Services
Related Products
References:
- Ahmad S, Punzi HA, Wright KN, Groban L, Ferrario CM. Newly developed radioimmunoassay for Human Angiotensin-(1–12) measurements in plasma and urine. Molecular and Cellular Endocrinology. 2021;529:111256. doi:10.1016/j.mce.2021.111256
- Rasmi RR, Kadwad VB, Sarnaik J, Shenoy KB, Somashekarappa HM. Development of radioimmunoassay for estimation of C-peptide in human serum. J Radioanal Nucl Chem. 2021;327(2):923-928. doi:10.1007/s10967-020-07536-4
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

