Physical & Chemical Index Test Service
Background on Physical and Chemical Parameter Testing in Food Products
The Physical & Chemical Index Test is a comprehensive analytical method used to evaluate the quality, safety, and characteristics of various products, particularly in the fields of food, pharmaceuticals, cosmetics, and materials science. This type of testing combines both physical and chemical analyses to provide a holistic assessment of a product's properties. It is essential for ensuring compliance with regulatory standards, verifying product specifications, and maintaining quality control throughout the manufacturing process.
Physical indexes describe the observable and measurable characteristics of a substance, such as color, melting point, boiling point, volatility, solubility, density, electrical conductivity, moisture content, osmotic pressure, and purity. These properties provide a clear and direct understanding of the product’s physical nature and are often essential for quality control, formulation, and processing decisions.
Chemical indexes , on the other hand, offer insight into the product’s behavior at a molecular level. These include parameters such as stability, flammability, acidity, alkalinity, oxidizing/reducing potential, crystal form, and pH. Unlike physical properties, chemical indexes typically require specialized testing methods and instrumentation. These tests are particularly valuable for evaluating a substance’s safety, reactivity, and long-term efficacy—especially in pharmaceuticals and other regulated industries.
At Creative BioMart , we specialize in decoding this data. With cutting-edge technology and expert analysis, we provide comprehensive, accurate, and reproducible physical and chemical index testing services. Whether you’re ensuring regulatory compliance or optimizing formulation, we’ve got your sample covered—literally from the inside out.
Our Comprehensive Physical and Chemical Index Test Services
Service Procedure

Service Details
Our testing capabilities span a wide range of physical and chemical properties. Here’s a sneak peek of what we can measure—and how:
|
Category |
Items |
Methods |
|---|---|---|
|
Physical Indexes |
Color, Melting/Boiling Point, Density, Moisture, Solubility, Purity, Conductivity |
Moisture analyzer, DSC, TGA, HPLC, LC-MS/MS, etc. |
|
Particle Size, Osmotic Pressure, Hygroscopicity, Phase Transitions |
Particle size analyzer, osmotic pressure tester, DVS, DSC |
|
|
Chemical Indexes |
pH, Stability, Flammability, Acidity, Redox Properties, Peroxide/Hydrogen Peroxide Values |
pH meter, biochemical assays, TGA |
|
Content Uniformity, Decomposition, Solvent Residue, Elemental Analysis |
ICP-MS, HS-GC-MS/MS, UV, PDA, ELSD detection |
|
|
Chiral Analysis, Dissolution Rate, Disintegration Time |
HPLC-UV, GC, dissolution/disintegration testers |
Advantages of Our Physical and Chemical Index Test Service
- Comprehensive Coverage: From melting point to molecular stability—we test it all, with no property left behind.
- High-Tech, High-Accuracy: Our labs are equipped with industry-leading instruments like LC-MS/MS, ICP-MS, HPLC, GC, and more.
- Expert Interpretation: Our scientists don’t just hand over numbers—they help you understand what they mean for your product.
- Tailored Solutions: Whether you’re developing a new drug or checking your food product's shelf-life, we customize the test plan to your goals.
- Regulatory Support: We ensure your results meet global standards—FDA, EMA, ICH, you name it.
- Responsive Customer Support: Fast replies. Clear answers. Friendly faces (well, voices/emails at least). We're with you at every step.
Case Studies on Physical and Chemical Index Test
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Silica dry coprocessing enhances blend flow and drug uniformity
Kim et al. , 2023. doi:10.1016/j.xphs.2023.05.016
This study investigated how dry coating two fine APIs—acetaminophen (mAPAP) and ibuprofen (Ibu)—affects their 30 wt% blends with fine excipients. Dry coating with hydrophobic silica (R972P) improved blend flowability, bulk density, and reduced agglomeration, resulting in better blend uniformity (BU). Uncoated APIs had poor flow and unacceptable BU. Coated blends also showed enhanced dissolution despite the hydrophobic coating, likely due to reduced API agglomeration and improved particle dispersion.
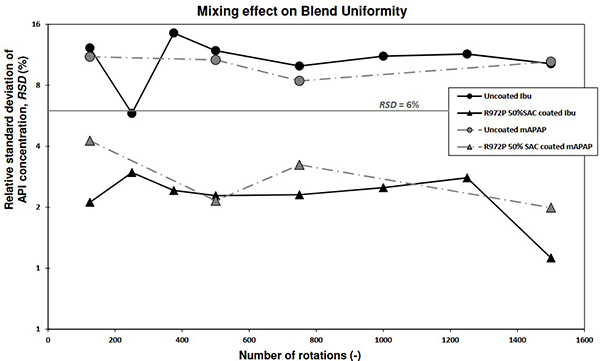
Figure 1. Content uniformity (RSD) of the blends as a function of number of rotations for two APIs. The solid grey line signifies the target % RSD to discern the uniformity of the blends. (Kim et al. , 2023)
Case 2: Impact of particle size on physical and functional characteristics of baby corn powder
Akbar et al. , 2025. doi:10.55730/1300-011X.3250
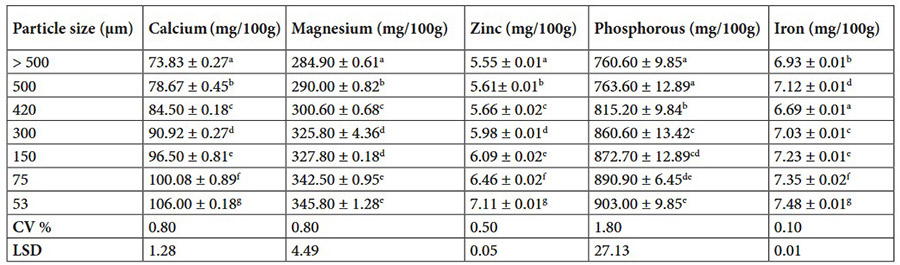
This study investigated how particle size affects the characteristics of baby corn powder. Sieve analysis revealed a size range from >500 µm to 53 µm, with smaller particles showing lower bulk density and better flowability. Antioxidant activity strongly correlated with phenolic and flavonoid content. Principal component and clustering analyses grouped the samples based on functional and nutritional traits, highlighting that 300 µm particles exhibited distinct biochemical and techno-functional advantages ideal for food applications.
Table 1. Mineral composition of baby corn powder at various particle sizes. (Akbar et al. , 2025)
Client Feedback on Our Physical and Chemical Testing Services
“We relied on Creative BioMart for a full suite of physical and chemical index tests during the formulation phase of a new oral drug candidate. Their team provided incredibly detailed data on dissolution rate, pH stability, and moisture sensitivity—all of which helped us fine-tune our excipient selection.”
— Head of Preclinical Formulation | Mid-Sized Pharmaceutical Company
“We engaged Creative BioMart to assess the purity and solvent residue levels in several batches of a new food-grade additive. Their HS-GC-MS/MS and HPLC analysis not only met our internal QA benchmarks but helped us identify a trace impurity we would have otherwise missed. Their technical staff even suggested an alternative testing parameter that improved our shelf-stability model.”
— Director of Product Quality | Food Ingredients Manufacturer
“Our R&D group needed rapid analysis on a botanical extract under accelerated stability testing. Creative BioMart performed osmotic pressure, peroxide value, and chiral analysis with precision. Their use of DVS for hygroscopicity testing was particularly valuable for determining optimal packaging. We’ll definitely continue using their services for all compound characterization going forward.”
— Senior Research Scientist | Natural Products Division, Nutraceutical Company
“As part of our tech transfer process for a generic API, we needed comprehensive batch-to-batch uniformity data including particle size distribution, acid value, and polymorphism. Creative BioMart delivered a complete package of results using ICP-MS, DSC, and X-ray diffractometry. Their ability to translate complex results into actionable insights made our regulatory filing significantly smoother.”
— Regulatory Affairs Manager | Global Generic Pharmaceutical Manufacturer
Frequently Ased Questions (FAQs)
-
Q: What types of samples can you test?
A: We can test a wide variety including pharmaceuticals, food products, industrial chemicals, cosmetics, and more. -
Q: How long does the testing take?
A: It depends on the test panel, but most standard analyses are completed within 5–10 business days. -
Q: Can I choose specific tests instead of a full panel?
A: Absolutely. We offer both full-spectrum analysis and à la carte testing based on your needs. -
Q: Are your results compliant with regulatory standards?
A: Yes, all results are delivered according to global standards such as USP, EP, JP, and ICH guidelines. -
Q: How do I send my samples?
A: We’ll provide detailed instructions, including sample amount, packaging, and shipping conditions. -
Q: Will I get help interpreting the results?
A: Definitely. Our experts will walk you through the report and help you make sense of the numbers.
Resources
Related Services
References:
- Akbar U, Rasane P, Mondol MSA, et al . Impact of particle size on physical and functional characteristics of baby corn powder. Turkish Journal of Agriculture and Forestry . 2025;49(1):75-88. doi:10.55730/1300-011X.3250
- Kim SS, Seetahal A, Amores N, Kossor C, Davé RN. Impact of silica dry coprocessing with API and blend mixing time on blend flowability and drug content uniformity. Journal of Pharmaceutical Sciences . 2023;112(8):2124-2136. doi:10.1016/j.xphs.2023.05.016
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

