GLP-Compliant Bioprocess Services
Understanding GLP Requirements in Preclinical Bioprocess Development
Good Laboratory Practice (GLP)-compliant bioprocesses are essential in ensuring the quality, reliability, and integrity of non-clinical safety studies, particularly in the pharmaceutical and biotechnology industries. GLP is a set of principles that govern the planning, performance, monitoring, recording, and reporting of laboratory studies, providing a framework to generate data that can be trusted for regulatory submissions.
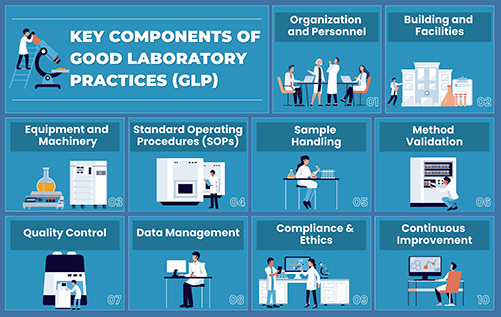
Figure 1. Key components of good laboratory practice (GLP).
Key Components of GLP Compliance
GLP compliance involves several critical components:
- Organization and Personnel
- Facilities and Equipment
- Standard Operating Procedures (SOPs)
- Quality Assurance
- Documentation and Record Keeping
Application in Bioprocessing
In bioprocessing, GLP is particularly important during the non-clinical phase, where safety and efficacy tests are conducted. This includes the handling of biological materials, the performance of in vitro and in vivo studies, and the generation of pharmacokinetic and toxicology data. GLP-compliant bioprocess services often involve:
- GLP-compliant procedures for transient protein expression, stable cell line production, and process development.
- Continuous evaluation and improvement of processes to maintain high standards.
- Proper maintenance and control of facilities and equipment.
Creative BioMart offers GLP-Compliant Bioprocess Services for the production of recombinant proteins and therapeutic antibodies, supporting in vivo studies and enabling seamless integration of data into manufacturing workflows and regulatory submissions.
Our GLP-Compliant Bioprocess Development and Testing Service Offering
Service Procedure

Service Highlights
We offer a full spectrum of GLP-Compliant Bioprocess Services tailored to meet your study design and regulatory goals, including:
-
GLP-Compliant Transient Protein Expression
- Rapid production of research-grade proteins for non-clinical studies
- Scalable expression in HEK293 or CHO cells under GLP guidelines
-

GLP-Compliant Research Cell Line Package
- Generation and characterization of research-use cell lines
- Documentation and traceability aligned with GLP protocols
-
GLP-Compliant Stable Cell Line Production
- Creation of production-level stable cell lines for recombinant protein or antibody expression
- Comprehensive data packages for regulatory submission
-
GLP-Compliant Process Development
- Upstream/downstream process optimization
- Technology transfer support under GLP-aligned documentation practices
Key Elements of Our GLP System
- Dedicated Project Teams : Staffed with GLP-trained scientists, project managers, and QA personnel.
- Facility & Equipment Control : Strict maintenance, calibration, and qualification of all instruments and manufacturing spaces.
- Material Management : Controlled receiving, handling, labeling, and release of raw materials and final deliverables.
- Process and Documentation Control : Batch records, SOPs, deviation management, and change controls aligned with GLP principles.
- Continuous Quality Improvement : Ongoing review and refinement of workflows, training, and risk mitigation strategies.
Why Our QC Services Ensure Consistent Product Safety and Efficacy
- Extensive GLP Expertise : Proven track record in recombinant protein and antibody production under GLP standards.
- Regulatory Confidence : Comprehensive documentation and QA oversight to support compliance.
- Flexible Service Packages : Customizable solutions for early research to preclinical development.
- Seamless Study Integration : Compatible with tox, PK, and IND-enabling workflows.
- Transparent Project Management : Clear timelines, milestone tracking, and open communication.
- End-to-End Support : From development to production, ensuring full regulatory alignment.
Case Studies on Preclinical Bioprocess Development Under GLP
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Validated bioanalytical assays for a novel ADC
Hou et al. , 2025;131:107580. doi:10.1016/j.vascn.2024.107580
This GLP-compliant study focused on validating two ligand binding assays for quantifying GQ1001—an antibody-drug conjugate (ADC) composed of Trastuzumab site-specifically linked to DM1—in cynomolgus monkey serum. Following regulatory bioanalytical method validation guidelines, both assays (for conjugated and total GQ1001) met predefined acceptance criteria for precision, accuracy, and reproducibility. No matrix or hemolysis effects were observed, and the assays showed strong dilution linearity and no hook effect up to 20.2 mg/mL. GQ1001 was stable in monkey serum during sample handling and storage. Incurred sample reanalysis (ISR) and parallelism assessments confirmed assay robustness. These validated methods were then successfully applied in a single-dose pharmacokinetic (PK) study, allowing reliable measurement of GQ1001 serum concentrations and exposure over time. The results support the use of these GLP-compliant assays in nonclinical development of ADCs like GQ1001.
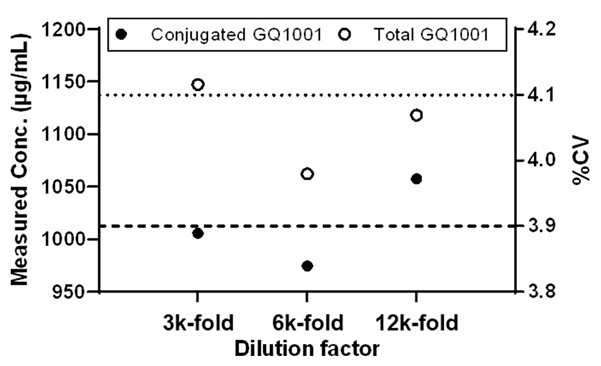
Figure 2. Parallelism evaluation. The parallelism investigation was conducted by using a real TK sample diluted with three dilution factors (3000, 6000 and 12,000-fold), and the dotted line and dashed line represent the %CV of measured concentrations of the conjugated GQ1001 and that of measured concentrations of the total GQ1001, respectively. (Hou et al. , 2025)
Case 2: GLP-compliant preclinical study of 3D-cultured MSCs for spinal fusion
Cho et al. , 2022. doi:10.1093/stcltm/szac052
Spinal fusion surgery aims to stabilize the spine by fusing vertebrae, but despite advances like synthetic bone substitutes and recombinant proteins, high revision rates due to pseudarthrosis persist. To address this, 3D spheroids of human bone marrow-derived mesenchymal stem cells (MSCs) were applied in a mouse spinal fusion model, as conventional 2D cultures compromise stem cell efficacy. A scalable, GMP-applicable manufacturing platform was developed for MSC spheroids, supported by biomolecular analyses such as LC-MS and bioinformatics to establish quality control standards for identity, purity, viability, and potency. In vivo , both undifferentiated and osteogenically induced MSC spheroids significantly enhanced angiogenesis, bone regeneration, and mechanical strength compared to 2D-cultured MSCs. For future clinical use, the safety of 3D MSC spheroids remains a critical consideration. Therefore, GLP-compliant toxicology studies are recommended, to be performed by certified nonclinical CROs, to ensure safe translation into spinal fusion therapies.
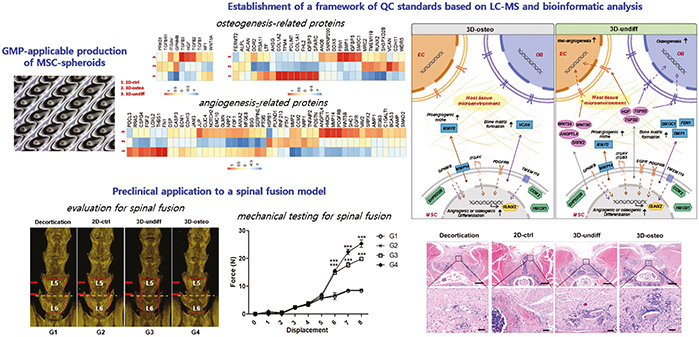
Figure 3. Preclinical study of human bone marrow-derived mesenchymal stem cells using a 3-dimensional manufacturing setting for enhancing spinal fusion. (Cho et al. , 2022)
Client Experiences with Our GLP Bioprocess Solutions
“We needed GLP-compliant transient protein expression in HEK293 cells for our in vivo tox studies on a bispecific antibody candidate. Creative BioMart not only delivered a high-yield prep on schedule but also ensured full traceability and documentation for our regulatory submission. Their QA support during our audit prep was outstanding.”
— Director of Non-Clinical Development | Mid-Sized Biopharmaceutical Company
“Our team was up against a tight deadline for a stability study requiring a recombinant enzyme produced under GLP standards. Creative BioMart scaled up production efficiently and delivered a validated product with full batch records, allowing us to hit our IND filing window without compromise.”
— Senior Project Manager | Global CRO Partnering in Metabolic Disorders
“We outsourced the generation of a GLP-compliant stable CHO cell line for our Fc-fusion protein. Creative BioMart’s documentation was meticulous, and their team worked seamlessly with our internal QA staff. They even identified an upstream optimization that improved our yield by 30%.”
— Principal Scientist | Biotech Company Focused on Rare Diseases
“We had difficulty finding a partner to deliver a research-grade protein under full GLP compliance. Creative BioMart’s team not only met our technical specs but went above and beyond to clarify regulatory expectations for our biocide submission. Their team is as sharp as they are collaborative.”
— Regulatory Affairs Manager | Agrochemical and Life Sciences Company
FAQs on GLP-Compliant Bioprocessing for Early-Stage Programs
-
Q: What types of molecules can you produce under GLP-compliant conditions?
A: We specialize in the production of recombinant proteins and therapeutic antibodies using transient and stable expression systems (e.g., CHO, HEK293) tailored for in vivo studies, toxicology testing, and IND-enabling applications. -
Q: What documentation do you provide to support regulatory submissions?
A: We deliver a complete GLP-compliant study package, including validated batch records, analytical data, quality control results, and a comprehensive QA-reviewed final report—fully aligned with regulatory expectations (FDA, EMA, etc.). -
Q: Do you offer flexible service models based on our development stage?
A: Yes, we offer modular service packages to support early research, preclinical development, and full IND-readiness. Whether you need rapid protein expression or a full process development program, we tailor our services to your needs. -
Q: How do you ensure GLP compliance throughout the bioprocess?
A: Our operations are governed by strict GLP protocols, including validated SOPs, controlled raw material handling, ongoing QA oversight, and secure archiving. We maintain traceability from initial material sourcing to final product delivery. -
Q: Can you integrate with our downstream studies like toxicology and pharmacokinetics?
A: Absolutely. Our bioprocess services are designed for seamless integration with nonclinical workflows, enabling smooth data continuity into your tox, PK, and safety studies—saving you time and regulatory risk. -
Q: What’s your typical turnaround time for GLP-compliant production?
A: Turnaround depends on the complexity of the project, but we pride ourselves on delivering within tight timelines. For transient expression projects, delivery can be achieved in as little as 4–6 weeks from project kickoff.
Resources
Related Services
- Protein Expression and Purification Services
- Mammalian Expression Systems
- Bacterial Expression Systems (E. coli / Bacillus)
- Yeast Expression Systems
- Baculovirus-Insect Cell Expression
- Serum-Free Expression System
- Stable Cell Line Services
- GMP Stable Cell Line Development Platform
- cGMP Cell-Based Potency Assays
Related Products
References:
- Cho S, Choi H, Jeong H, et al . Preclinical study of human bone marrow-derived mesenchymal stem cells using a 3-dimensional manufacturing setting for enhancing spinal fusion. Stem Cells Translational Medicine . 2022;11(10):1072-1088. doi:10.1093/stcltm/szac052
- Hou Y, Miao J, Sun Y, et al . Ligand-binding assays validated for quantitative bioanalysis of a novel antibody-drug conjugate in monkey serum and related application in a nonclinical study. Journal of Pharmacological and Toxicological Methods . 2025;131:107580. doi:10.1016/j.vascn.2024.107580
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

