Pharmaceutical Product Release Testing
The Role of Product Release Testing in Pharmaceutical Quality Control
Pharmaceutical product release testing represents a critical and final quality control step in the drug manufacturing lifecycle. Following extensive research and development, scale-up, and production, release testing determines whether a pharmaceutical product meets the necessary standards for safety, efficacy, and consistency prior to market distribution.

Key Testing Categories
The core components of release testing typically include:
- Identity Testing: Verifies the presence of the correct active pharmaceutical ingredient (API) to ensure product authenticity.
- Potency (Assay): Quantifies the amount of API within the product to confirm it is within the specified range necessary for therapeutic efficacy.
- Purity and Impurity Profiling: Detects and quantifies potential impurities, including residual solvents, degradation products, and other contaminants.
- Microbial Limits Testing: Ensures that non-sterile products meet microbiological quality standards and are free from unacceptable microbial contamination.
- Sterility Testing: Confirms the absence of viable microorganisms in sterile products such as injectables and ophthalmic solutions.
- Dissolution and Disintegration Testing: Evaluates the rate at which oral solid dosage forms dissolve or disintegrate, which directly impacts drug bioavailability and therapeutic performance.
- Physical and Visual Inspection: Assesses the product’s appearance, color, texture, and other physical characteristics to detect any abnormalities or inconsistencies.
Creative BioMart is a cGMP-compliant, accredited quality control (QC) testing laboratory with a long-standing reputation for excellence in Pharmaceutical Release Testing. Whether you’re working with small molecules, inhalables, or cutting-edge biopharmaceuticals, we provide the rigorous analytical testing your product needs—without the long lead times.
Our Comprehensive Pharmaceutical Product Release Testing Services
Our services are designed to ensure your drug products meet compendial standards (USP, EP, JP, BP) and regulatory expectations while accelerating your path from manufacturing to market.
We are your reliable partner for:
- Batch release testing
- Lot release testing
- API, IMP, and finished product (FP) testing
- Compliance with global pharmacopeias
Service Procedure

Wide Testing Scope
-
Dosage Forms
Tablets (IR/SR), , Liquids, Suspensions, Injectables, Inhalers, Suppositories
-
Advanced Formats
Implantables, Ocular Implants, Contact Lenses, Synthetic blood
-
Delivery Systems
Patches, Inhalers, Controlled-release formulations
Technologies We Use
- Chromatography: LC/MS/MS, HPLC (UV, PDA, FLU, ELSD, RI), UPLC (TUV, PDA, SQD), ACQUITY UPLC H-Class
- Spectroscopy & Detection: GC, GPC, SEC, KF, TOC
- Mass Spectrometry: MALDI-TOF
- Aerosol Performance Testing: APSD (NGI, ACI)
Advantages of Our Pharmaceutical Product Release Testing
- Decades of Experience: Proven track record in all types of pharmaceutical testing.
- Speed Meets Precision: Fast turnaround without compromising quality.
- Regulatory Confidence: Our data is audit-ready and inspection-tested.
- Flexible Support: Customizable testing services to meet diverse project needs.
- Comprehensive Technology Suite: Equipped with advanced analytical platforms for broad testing capabilities.
- End-to-End Integration: Seamless link to development and manufacturing.
Case Studies: Real-World Success in Pharmaceutical Product Release Testing
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Controlled release of vitamin C via modified CNC/chitosan nanocapsules
Baek et al., 2021. doi:10.1016/j.crfs.2021.03.010
This study highlights the importance of robust pharmaceutical product release testing through the development and evaluation of Vitamin C (VC) nanocapsules designed for enhanced stability and controlled release. VC was encapsulated using glycidyl trimethylammonium chloride-chitosan (GCh) and cross-linked with phosphorylated cellulose nanocrystals (PCNC), forming stable nanocapsules with high encapsulation efficiency and sustained release over 14 days. Release kinetics followed first-order and Higuchi models confirming predictable drug release behavior. The nanocapsules also demonstrated broad-spectrum antibacterial activity. Pharmaceutical release testing under simulated gastrointestinal conditions and varying environmental factors showed that nitrogen purging and light protection significantly reduced VC degradation, preserving nearly 98% of its content over 5 days. In contrast, unprotected VC degraded completely within 4 days. These findings emphasize the critical role of comprehensive release testing and environmental control in ensuring accurate, stable, and functional delivery of active compounds in pharmaceutical formulations.
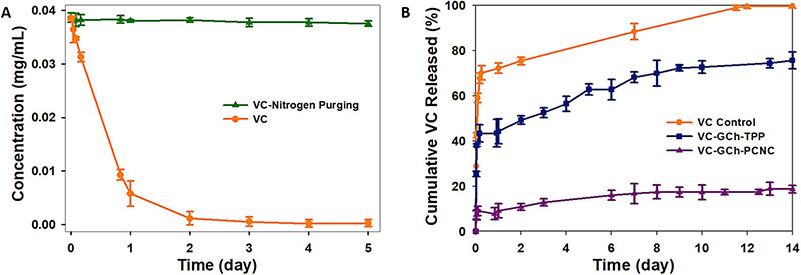
Figure 1. (A) Changes in concentration concerning the time of a 0.04 mg/mL VC solution in PBS under nitrogen gas purging, limited light exposure, and constant temperature. (B) The cumulative release profile of VC from nanocapsules through a dialysis membrane to time. (Baek et al., 2021)
Case 2: Controlled-release carbamide peroxide nanoemulgel for tooth bleaching
Okonogi et al., 2021. doi:10.3390/ph14020132
This study presents the development of a novel carbamide peroxide nanoemulgel (CP-NG) designed to control the burst release of CP, which in traditional hydrogels can cause periodontal inflammation. CP was first incorporated into solid dispersions using either white soft paraffin or a polyvinylpyrrolidone-paraffin mixture as carriers, which influenced its crystallinity. These dispersions were then used to formulate oil-in-water nanoemulsions, forming the basis of the CP-NG. The carrier type and CP-to-carrier ratio affected key properties such as particle size, zeta potential, and release behavior. The CP-NG followed a first-order release profile via an anomalous transport mechanism, with slower drug release observed as the carrier concentration increased. A 1:1 CP-to-polymer mixture ratio provided sustained release, high tooth whitening efficacy, and preserved enamel microhardness. This controlled-release formulation offers an effective and safer alternative to traditional bleaching agents, reducing inflammation risk while maintaining therapeutic performance.
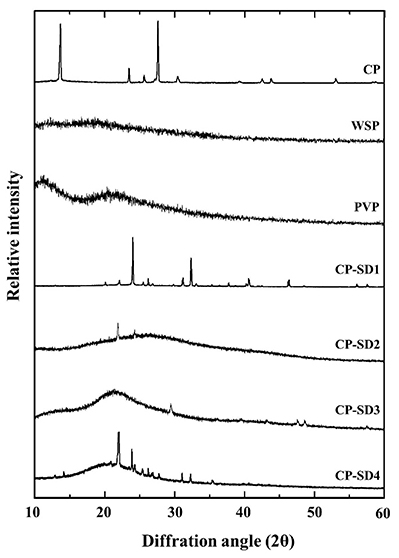
Figure 2. XRD patterns of CP-SD in comparison with different drug-carrier ratios and raw materials of CP and carriers. (Okonogi et al., 2021)
Client Feedback on Our Release Testing Support for Market Entry
"We had a tight regulatory deadline for the EU release of our sustained-release injectable. Creative BioMart completed full compendial testing, including dissolution, sterility, and impurity profiling, in under two weeks—without compromising quality. Their detailed documentation and responsiveness made our EMA submission process smooth and efficient."
— Director of Quality Assurance | Mid-Sized European Pharmaceutical Company
"Our team was struggling to validate an HPLC method for an inhalable peptide formulation. Creative BioMart not only optimized the gradient and detection parameters but also identified minor degradation products we had missed. Their technical acumen helped us avoid a potential delay in our clinical trial material release."
— Head of Analytical Development | Global Biopharmaceutical Manufacturer
"We selected Creative BioMart to handle batch release testing for our ophthalmic implant. The product required customized testing for extractables, particulate matter, and release kinetics. Their team demonstrated deep expertise in handling niche delivery systems and delivered results that were both compliant and highly reproducible."
— Principal Scientist | Specialty Drug Delivery Company
"During scale-up, we needed rapid lot release testing for multiple solid oral dosage forms. Creative BioMart handled assay, content uniformity, dissolution, and microbial testing across 12 batches with zero deviations. Their lab's efficiency and clear communication helped keep our manufacturing timeline intact."
— Director of Manufacturing Operations | Generic Pharmaceutical Firm
Frequently Asked Questions (FAQs) on Pharmaceutical Product Release Testing
-
Q: What types of pharmaceutical products do you test?
A: We support a wide range of products including tablets, capsules, liquids, injectables, inhalers, suppositories, implants, and more—across both small molecule and biopharmaceutical pipelines. -
Q: Are you compliant with regulatory standards?
A: Absolutely. We operate under full cGMP compliance and align our testing protocols with USP, EP, JP, BP, and ICH guidelines. -
Q: What’s your typical turnaround time?
A: We understand the urgency of product release. Timelines vary based on complexity, but we aim to deliver results efficiently while maintaining the highest quality standards. -
Q: Can you handle custom method development?
A: Yes! Our scientists are adept at developing and validating tailored analytical methods when compendial ones aren’t applicable.
Resources
Related Services
- Downstream Processing Development
- Biosimilar Comparability Studies
- cGMP Cell-Based Potency Assays
- Quality Control
- Pharmaceutical Stability Analysis
- Protein Analytical Service
- Endotoxin Removal Service
- Microbiological Test Service
- Biopharmaceutical Process and Product Related Impurities Analysis
Related Products
References:
- Baek J, Ramasamy M, Willis NC, Kim DS, Anderson WA, Tam KC. Encapsulation and controlled release of vitamin C in modified cellulose nanocrystal/chitosan nanocapsules. Current Research in Food Science. 2021;4:215-223. doi:10.1016/j.crfs.2021.03.010
- Okonogi S, Kaewpinta A, Khongkhunthian S, Chaijareenont P. Development of controlled-release carbamide peroxide loaded nanoemulgel for tooth bleaching: in vitro and ex vivo studies. Pharmaceuticals. 2021;14(2):132. doi:10.3390/ph14020132
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

