Potency Assay Kits

🧪 Kit-1786
Source:
Species:
Tag: Non
Conjugation:
Protein Length:
Background
Overview
Potency assay kits are a critical part of evaluating the efficacy of treatments against the targets they develop. This type of kit is based on the principle of biochemical reaction to quantitatively analyze the concentration of drugs in the organism, which helps to quickly study the therapeutic effect.
These kits are designed based on several different technology platforms, the most common of which is enzyme-linked immunosorbent assay (ELISA). The followings are the principles of several potency assay kits:
ELISA (Enzyme-linked immunosorbent assay) : This is an immunological method that relies on the principle of antigen-antibody interactions and utilizes enzyme and colorimetric assents to quantify target molecules. ELISA assays can be designed in different formats, such as direct ELISA, indirect ELISA, sandwich ELISA, and competitive ELISA, each with its specific applications and advantages.
Chemiluminescence immunoassay: This method produces a light signal through a chemical reaction and is used to detect drug concentrations. It has the advantages of high sensitivity and high degree of automation, and is suitable for high-throughput testing in clinical laboratories.
Fluorescence polarization immunoassay (FPIA) : This method uses fluorescently labeled antigens to compete with antigens in the specimen to bind specific antibodies. By measuring the intensity of polarization fluorescence, the concentration of the drug in the specimen can be determined, because the complex labeled antigen and antibody rotates slowly with large molecular weight, and emits strong polarization fluorescence; The molecular weight of the free labeled antigen is small, the rotation is fast, and the polarization fluorescence is weak.
Liquid chromatography-Tandem mass spectrometry (LC-MS/MS) : This is a technique that combines liquid chromatography and mass spectrometry to provide highly specific and sensitive quantitative analysis for drug concentration determination in complex biological samples.
Luminex xMAP technology: This is a multi-assay technology based on fluorescent-encoded microspheres that can simultaneously detect a number of different biomarkers. It combines fluorescent coding microspheres with specific antibodies or probes for analysis by flow cytometry and is suitable for high throughput analysis.
Target Types
The target classification of the potency assay kits usually involves the type of molecule that the drug acts on, which can be proteins, enzymes, receptors, ion channels, etc. Here are some common target categories:

Proteins: Many drugs work by binding to specific proteins, which may be enzymes, transcription factors, or structural proteins.
Enzymes: Enzymes are a special class of proteins that catalyze biochemical reactions. Drugs can affect physiological processes by inhibiting or activating enzyme activity.
Receptors: Receptors are large molecules on the surface or inside of cells that can bind to specific ligands (e.g., hormones, neurotransmitters) and trigger cell signaling.
Ion channels: Ion channels are pores in the cell membrane that allow specific ions to pass through, maintain ion balance inside and outside the cell, and are critical for processes such as nerve signaling and muscle contraction.
Transporters: These proteins are responsible for the transport of substances inside and outside the cell, including drugs, metabolites, and nutrients.
Genes and RNA: Certain drugs may act directly or indirectly on genes or RNA, for example by interfering with RNA splicing or stability to affect protein expression.
Cytokines: Cytokines are a class of signaling proteins that are involved in communication between cells, especially in immune responses and inflammatory responses.
Growth factors: Growth factors are proteins that stimulate cell growth and division, and they play a key role in tissue repair and regeneration.
Tumor markers: In cancer therapy, changes in the expression of specific proteins or genes may serve as targets for drug action.
Metabolites: Drugs may affect specific products in metabolic pathways that regulate the metabolic activity of cells.
Development Status
Potency assay kits are becoming more widely used in clinical pharmacy and drug development, mainly due to the growing demand for personalized therapy and precision medicine, as well as continuous advances in analytical technology. Here are a few key points about the status of potency assay kits:
In vivo drug analysis technology has become one of the important auxiliary technologies to promote clinical rational drug use, improve the level of individualized treatment, and reduce the occurrence of adverse drug reactions. At present, in clinical or scientific research development, the means of drug efficacy evaluation and the development of related technologies are also very diverse, including high performance liquid chromatography (HPLC), liquid chromatograph-mass spectrometry (LC-MS/MS), immunological detection technology (IA), biosensing technology and capillary electrophoresis (CE).
At the same time, as the scale of global testing kits gradually expands, emerging markets and product types and technologies will also develop simultaneously. Although the potency assay kit has made some progress, it still faces some challenges in practical application, including how to improve the sensitivity and accuracy of the detection, simplify the sample pretreatment process, shorten the detection time and reduce the cost. Future research may focus on developing non-invasive or minimally invasive sampling strategies, improving the interpretation of drug monitoring results, developing new techniques for more accurate and sensitive in vivo drug analysis, and further optimizing existing detection methods.

Applications
Therapeutic drug monitoring (TDM) : Determination of drug concentration to ensure the safety and efficacy of drug therapy, especially in situations where strict concentration control is required, such as immunosuppressants, antiepileptics, and antibiotics.
Pharmacogenomics research: Combining genetic testing to assess an individual's ability to metabolize and respond to specific drugs to guide personalized medicine.
Drug development: In drug development, potency assay kits are used to evaluate the pharmacokinetic properties and pharmacodynamics of new drugs to help optimize drug dosage and treatment regimen.
Immunogenicity assessment: For biotherapeutic drugs, testing drug levels and anti-drug antibody (ADAs) levels is essential to assess the immunogenicity and therapeutic effect of the drug.
Companion diagnostics: Development of companion diagnostic kits associated with specific drugs to help screen the patient population most likely to benefit from treatment and improve drug response and safety.
Case Study
Case Study 1: Notch1/CSL Reporter Kit (Kit-1786)
The family of Notch proteins plays a key role in cell fate determination. Additionally, Notch proteins regulate critical functions of the endothelium, as well as other recruited supporting cells, in concert with other pathways. Despite significant advances in the field and extensive studies focused on elucidating this pathway, many questions remain regarding Notch activation and its upstream/downstream effects, with vascular biology constituting one area of particular interest. Here, the researchers provide a brief description of the components and functions of the Notch pathway in vasculature, followed by a detailed compilation of recommended methods of evaluation in vitro and in vivo. They provide a rationale for key elements when choosing different approaches and controls, strengths and limitations, and essential considerations when providing a meaningful interpretation of results. This study aims to describe a careful approach to assessing Notch function in endothelial cells, based on underlying principles, with the overall goal of obtaining physiologically relevant information that will enhance our understanding of this pathway and its role in vascular biology.
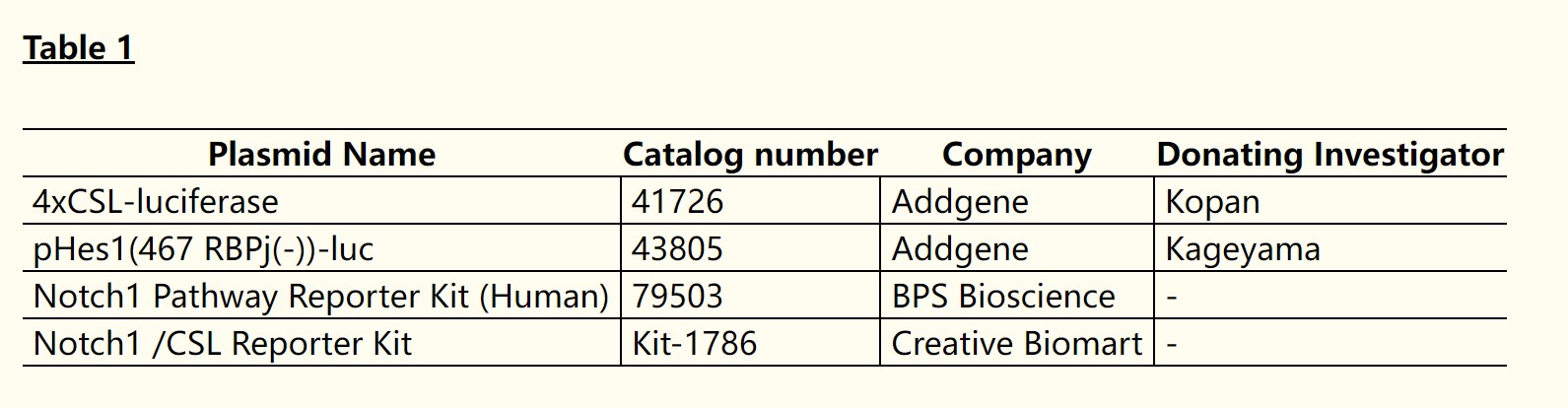
Table 1. Some luciferase constructs that can be used to detect Notch activation in vitro. (Lydia L Wu, 2021)
Case Study 2: Total Protein Assay Kit (Kit-0731)
Nephrotic syndrome (NS) is one of the most commonly encountered glomerular diseases in children and a major contributor to the workload of pediatric nephrologists. Nephrotic syndrome is a chronic disease that is well known for its long term serious complications. among these complications albuminemia and dyslipidemia. Many studies has related albuminemia and dyslipidemia to other complications of Nephrotic syndrome as retinopathy and cardiovascular disease of nephrotic syndrome. The main objective of this study was planned to elucidate the potential diagnostic role of transforming growth factor-beta (TGF-ß) as a novel biomarkers and its correlation with albuminemia and dyslipidemia in nephrotic syndrome among Egyptian children. Serum level of TGF-ß was measured using ELISA, serum level of lipid profile and urinary proteins were estimated. This study revealed that serum level of TGF-ß was higher in nephrotic cases than controls and its serum level was significantly negative correlated with serum albumin while it showed a significant positive correlation with urinary protein and lipid indices levels in children with nephrotic syndrome.
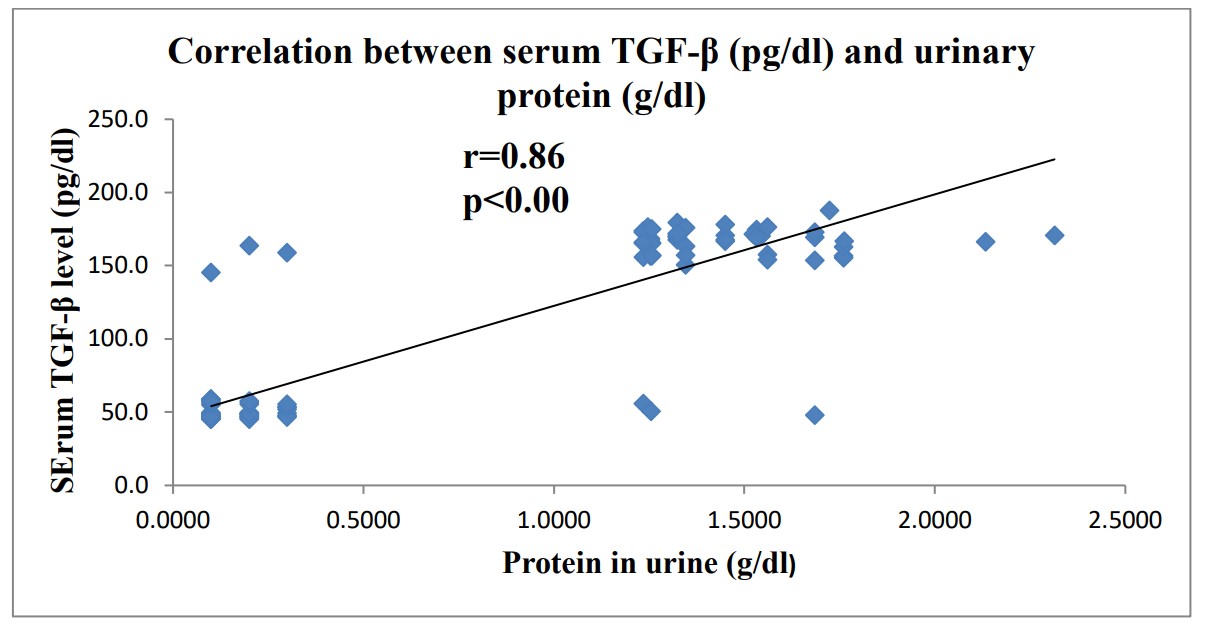
Fig2. Correlation between Serum TGF-β and Urinary Total Protein. (Faten Z. Mohamed, 2019)
Case Study 3: Creatinine Assay Kit
Hugan Buzure Granule (HBG) is a traditional prescription of Uygur nationality in China mainly used to treat liver cold, stomachache, spleen and rib pain, arthralgia, rheumatism and urinary system diseases. Its mechanism of action in treating acute kidney injury (AKI) continues to remain unconfirmed. This study’s objective was to investigate the pharmacodynamics and mechanism of HBG in the management of AKI. The damage to the kidney tissue was examined by using H&E (Hematoxylin-eosin) staining. The BUN (Blood Urea Nitrogen) and Cr (Creatinine) in serum were examined by biochemical kit. ELISA kit was utilized to assess the content of IL-6, TNF-α, and IL-1β in serum. Immunohistochemistry and Western blot were utilized to identify the translation of proteins associated to the NLRP3/Caspase-1 pathway and the TLR4/NF-κB pathway in various tissues. The results showed HBG considerably improved the renal injury in mice and decreased their kidney coefficient in contrast with the Control group.
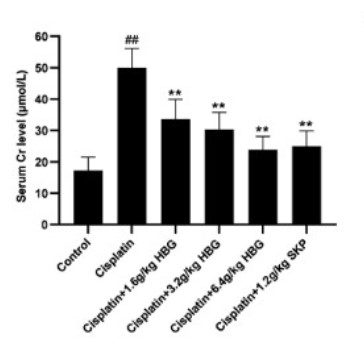
Fig3. Serum Creatinine (Cr). (Chongwang Ran, 2023)
Potency assay is a critical part of evaluating the efficacy of a therapy against a target it has developed. By using our kits to quantitatively analyze the concentration of a drug in a living organism based on the principles of biochemical reactions, we can help quickly study the effect of your treatment. If you have any other customized needs, please feel free to contact us!















