Enzyme Immobilization
Creative BioMart offers a cutting-edge Enzyme Immobilization Service using a proprietary platform that combines affinity-tag technology with inert porous materials. Our method enables single-step purification and immobilization from cell lysates or supernatants, delivering robust, recyclable, and high-performing immobilized enzymes suitable for biocatalysis in organic solvents or aqueous buffers. With enhanced stability and streamlined workflow, our enzyme immobilization solution is ideal for both research and industrial-scale applications—whether for lactose-free milk production or pharmaceutical synthesis.
Understanding Enzyme Immobilization and Its Role in Biocatalysis
Enzyme immobilization is a technique that involves attaching enzymes to a solid support or encapsulating them within a matrix. This process transforms the enzymes from a freely soluble form to a fixed form. The immobilized enzymes retain their catalytic activity and can be used repeatedly in various biotechnological processes.
Reasons for Enzyme Immobilization
Traditionally, soluble enzymes have been limited by poor stability and challenging downstream processing. Immobilized enzymes address these limitations by:
- Enhancing thermal, chemical, and operational stability, thus prolonging enzyme lifespan.
- Simplifying product purification, as the enzyme remains fixed and can be easily separated from the reaction mixture.
- Enabling enzyme reuse, significantly reducing biocatalyst costs in repetitive or continuous processes.
- Allowing for better control of catalytic activity through spatial orientation and density regulation.
Methods of Enzyme
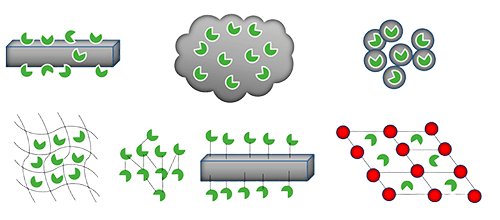
- Adsorption : This is a relatively simple method where enzymes are physically adsorbed onto the surface of a solid support. The support materials can include activated carbon, silica gel, and ion-exchange resins.
- Covalent Bonding : In this method, enzymes are chemically bonded to the support material through covalent bonds. The support can be modified with functional groups such as aldehyde, epoxy, or carboxyl groups that can react with amino, hydroxyl, or thiol groups on the enzyme molecules.
- Entrapment : Enzymes can be entrapped within a polymer matrix. The matrix can be made of natural or synthetic polymers such as alginate, chitosan, or polyacrylamide.
- Cross-linking : Enzyme molecules can be cross-linked with each other or with other proteins to form a stable network. This can be done using cross-linking agents such as glutaraldehyde.
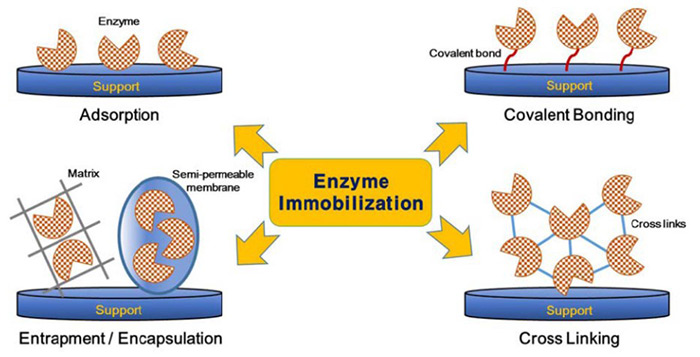
Figure 1. Various methods of enzyme immobilization. (Nguyen and Kim, 2017)
Creative BioMart has developed a revolutionary platform that allows the one-step immobilization of affinity-tagged enzymes directly from crude lysates or culture supernatants. Our system uses inert porous materials to support a wide variety of enzymatic functions in organic solvents or aqueous buffers, without compromising performance or accessibility.
This streamlined approach is ideal for applications such as:
- Food processing (e.g., lactose-free milk production)
- Pharmaceutical biocatalysis
- Diagnostic assays
- Green chemistry and biotransformation
Custom Enzyme Immobilization Services for Research and Industrial Applications
Service Procedure

Our Immobilization Methods
We offer four robust immobilization methods to match your specific needs:
Service Details
-
Affinity-tag Binding (Recommended)
High-efficiency method using non-covalent or covalent protein tags for site-specific immobilization onto porous matrices—ideal for stability and reusability.
-
Adsorption
Enzymes adhere non-covalently to glass or alginate supports. While easy to perform, enzyme activity can be hindered due to random orientations or blocked active sites.
-
Entrapment
Enzymes are enclosed in insoluble gels or beads (e.g., calcium alginate). Suitable for mild conditions, but mass transfer limitations may affect reaction speed.
-
Cross-linkage
Covalent linking of enzyme molecules into self-supporting matrices. Maintains activity while offering high durability. Spacers like PEG can reduce steric hindrance.
Service Details & Technical Options
-
Support Materials
Glass beads, porous matrices, alginate, or custom carriers
-
Tag Support
His-tag, GST-tag, FLAG-tag, and others
-
Reaction Compatibility
Suitable for both organic solvents and aqueous buffers
-
Product Formats
Beads, columns, or bulk slurry depending on downstream use
-
Scale
From research scale to pilot production batches
Custom Solutions
-
❔ Need help tagging your enzyme?
😊 We offer custom cloning and expression support.
-
❔ Have a proprietary substrate or reaction condition?
😊 We’ll optimize for your system.
-
❔ Require documentation?
😊 We provide complete QC reports and technical protocols with each project.
Why Choose Our Enzyme Immobilization Platform for High Efficiency and Stability
- One-Step Purification & Immobilization : Directly immobilize from crude extract or supernatant—saving time, reagents, and effort.
- Enhanced Enzyme Stability : Our immobilized enzymes show improved thermal and operational stability, reducing enzyme degradation.
- Economical & Recyclable : Immobilized enzymes are easily separated and reused, lowering costs over repeated cycles—perfect for high-volume production like lactose-free dairy processing.
- Flexible Immobilization Strategies : Choose the method that best suits your enzyme and process conditions—from non-covalent affinity to covalent cross-linking.
- Easy Workup, Cleaner Products : Reactions with immobilized enzymes result in minimal residual enzyme, simplifying downstream purification and analysis.
- Broad Compatibility : Works in organic solvents and water-based systems—supporting a wide range of industrial and academic applications.
Real-World Applications: Enzyme Immobilization Case Studies Across Industries
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Immobilization of thrombin on agarose-based supports for affinity tag removal
Almada et al. , 2025. doi:10.1021/acs.biomac.5c01010
This study highlights the advantages of immobilizing thrombin; a key serine protease used for removing affinity tags in recombinant protein purification. By covalently attaching bovine thrombin to glyoxyl-activated agarose, researchers achieved enhanced enzyme stability, reusability, and efficiency. The immobilized thrombin successfully cleaved 6xHis-tags and other peptide tags with activity comparable to its soluble counterpart. Notably, it remained fully active after 1.5 hours at 50 °C and retained performance across 10 reuse cycles, achieving a space-time yield of 4.7 g/(L·h). These results demonstrate its value as a robust, reusable, and cost-effective biocatalyst for improved protein purification in both industrial and lab-scale settings.
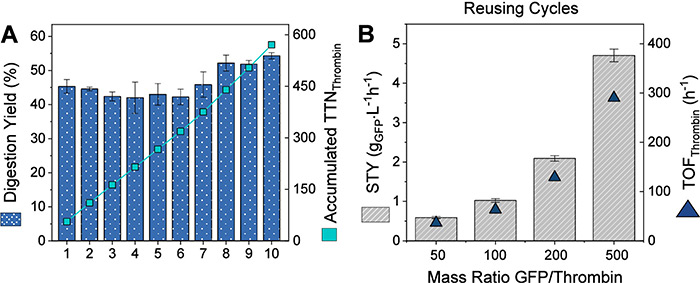
Figure 2. Enzyme Reusability and Process Efficiency. (A) Reusability of immobilized thrombin (THL@Gx) in repeated 6xHis-GFP digestion cycles; TTN calculated per cycle. (B) Process intensification at increasing substrate concentrations; STY and TOF reflect catalytic efficiency under scaled conditions. (Almada et al ., 2025)
Case 2: Enhanced α-glucosidase stability via cryogel entrapment in charged reactors
Demirci and Sahiner, 2021. doi:10.1016/j.cej.2020.128233
To overcome the limitations of enzyme cost, stability, and sensitivity, α-Glucosidase (α-Glu) from Saccharomyces cerevisiae was immobilized in various synthetic cryogels—neutral, anionic, and cationic—during synthesis. The resulting cryogels, α-Glu@p(AAm), α-Glu@p(AMPS), and α-Glu@p(APTMACl), retained 80.9%, 61.5%, and 50.9% of enzyme activity at optimal pH 6.8 and 37 °C. Notably, activity reached 100% at more extreme pH values, unlike free α-Glu, which became inactive. The immobilized forms also demonstrated superior reusability and storage stability, maintaining over 50% activity after 10 uses and 10 days at room temperature. Kinetic parameters (Km, Vmax) confirmed the improved performance.
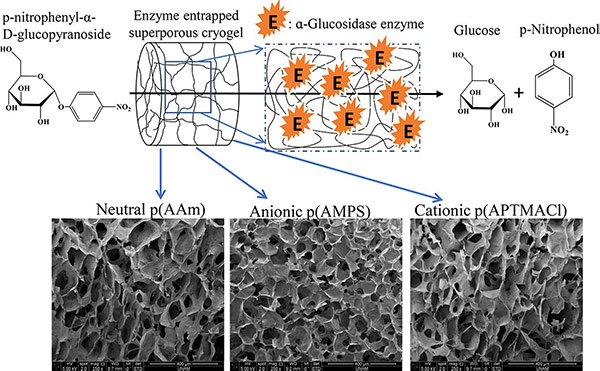
Figure 3. Superporous neutral, anionic, and cationic cryogel reactors to improved enzymatic activity and stability of α-Glucosidase enzyme via entrapment method (Demirci and Sahiner, 2021)
Customer Testimonials: Success with Our Immobilized Enzyme Solutions
“We partnered with Creative BioMart to immobilize a His-tagged oxidoreductase enzyme for a continuous-flow bioreactor system. Their affinity-tag binding method produced a remarkably stable enzyme preparation that retained over 90% activity after 15 reaction cycles. The one-step purification and immobilization process saved us weeks of work.”
— R&D Director | Mid-sized Biopharmaceutical Company
“Our project involved using immobilized lactase for lactose-free milk production. We needed a solution that could be reused repeatedly without enzyme loss or contamination. Creative BioMart’s cross-linkage immobilization strategy delivered exactly that. The enzyme matrix remains in the column while milk is processed, and we’ve already reused it over 25 times with consistent performance.”
— Process Development Scientist | Food Technology Firm
“We were exploring enzyme kinetics of a rare decarboxylase under non-aqueous conditions. Creative BioMart immobilized the enzyme using entrapment in calcium alginate microspheres, and the resulting prep-maintained activity even in organic solvents. Their team worked closely with us to fine-tune the protocol.”
— Principal Investigator | Academic Biochemistry Lab
“For our biotransformation workflow, we needed a robust and recyclable lipase system. Creative BioMart used adsorption onto silica microspheres, which turned out to be ideal for our solvent-based reactions. The immobilized lipase exhibited enhanced thermal stability and could be separated cleanly after reactions. The cost savings on enzyme reuse alone paid for the service.”
— Senior Scientist | Green Chemistry Startup
FAQs About Enzyme Immobilization Services, Techniques, and Applications
-
Q: What types of enzymes can be immobilized using your platform?
A: Our system supports a wide range of enzymes, particularly those that can be affinity tagged. Whether your enzyme originates from microbial, plant, or mammalian systems, we can tailor the immobilization strategy—including affinity-tag binding, entrapment, cross-linkage, or adsorption—to suit its characteristics and your application. -
Q: What are the benefits of enzyme immobilization in industrial biocatalysis?
A: Immobilized enzymes offer increased stability (thermal and operational), reusability, and easier product purification. This reduces costs and enhances process efficiency. For example, in lactose-free milk production, immobilized lactase remains in the container for repeated use, eliminating the need to repurify or re-add enzyme each cycle. -
Q: How does your service differ from standard enzyme purification?
A: Our platform integrates enzyme purification and immobilization in a single step—directly from a cell extract or culture supernatant. This saves time and reduces costs compared to multi-step purification and separate immobilization protocols. -
Q: Will enzyme activity be affected by immobilization?
A: Enzyme activity is carefully preserved by choosing the right immobilization method. Affinity-tag binding and cross-linkage with spacers like PEG help avoid steric hindrance and maintain accessibility of the active site. Additionally, our immobilized enzymes often show improved resistance to harsh conditions compared to their soluble counterparts. -
Q: What solvents or conditions are compatible with immobilized enzymes?
A: Our immobilized enzyme systems are designed to be versatile. They work in aqueous buffers or organic solvents, depending on your application. This makes them suitable for use in pharmaceutical synthesis, food processing, biofuels, and more. -
Q: Can I reuse the immobilized enzymes?
A: Yes! Reusability is one of the key economic advantages of immobilized enzymes. Our robust immobilization matrices allow multiple cycles of use without significant loss of activity, reducing enzyme consumption and production cost. -
Q: Do you provide quality control and technical support?
A: Absolutely. Each immobilization batch comes with complete QC documentation, including activity validation and coupling efficiency. Our technical experts are also available to help optimize the system for your specific biocatalytic process.
Resources
Related Services
- Lipid-based Protein Immobilization
- Enzyme Activity Assay
- Enzyme/Antibody conjugation
- Enzyme Target and Screening
- SUMO-tag Protein Production
- Affinity Chromatography
Related Products
References:
- Almada JC, Marco-Martin M, Roura-Padrosa D, Velasco-Lozano S. Immobilization of thrombin on agarose-based supports for affinity tag removal. Biomacromolecules . 2025;26(7):4718-4729. doi:10.1021/acs.biomac.5c01010
- Demirci S, Sahiner N. Superporous neutral, anionic, and cationic cryogel reactors to improved enzymatic activity and stability of α-Glucosidase enzyme via entrapment method. Chemical Engineering Journal . 2021;409:128233. doi:10.1016/j.cej.2020.128233
- Maghraby YR, El-Shabasy RM, Ibrahim AH, Azzazy HMES. Enzyme immobilization technologies and industrial applications. ACS Omega . 2023;8(6):5184-5196. doi:10.1021/acsomega.2c07560
- Nguyen HH, Kim M. An overview of techniques in enzyme immobilization. Appl Sci Converg Technol . 2017;26(6):157-163. doi:10.5757/ASCT.2017.26.6.157
- Zhao Z, Zhou MC, Liu RL. Recent developments in carriers and non-aqueous solvents for enzyme immobilization. Catalysts . 2019;9(8):647. doi:10.3390/catal9080647
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

