Non-Animal Skin Sensitization Testing Services
Allergic Contact Dermatitis (ACD) affects an estimated 15–20% of the global population, with cases steadily rising. Identifying the skin sensitization potential of chemicals is therefore a critical step in the safety evaluation of cosmetics, pharmaceuticals, and agrochemicals. At Creative BioMart, we provide comprehensive Non-Animal Skin Sensitization Testing Services using GLP-compliant methods. Our assays—Direct Peptide Reactivity Assay (DPRA), KeratinoSens™, and Human Cell Line Activation Test (h-CLAT)—are fully validated and OECD-accepted, enabling reliable prediction of sensitization potential while meeting ethical and regulatory requirements. We support our clients in integrating these assays into robust testing strategies for regulatory submissions.
Overview of Non-Animal Skin Sensitization Testing
The mechanism of skin sensitization has been investigated for decades and documented by the OECD as an adverse outcome pathway (AOP). In brief, the sensitizer covalently binds to the proteins of viable epidermal cells (key event 1), forming immunogenic hapten-protein conjugates. Meanwhile, keratinocytes become activated and release danger signals (key event 2). Next, dendritic cells change their phenotype by recognizing the hapten-protein conjugates and danger signals (key event 3). The activated dendritic cells then migrate from the skin to the draining lymph node, where they present the allergen to T cells. After binding to a hapten-peptide-specific T cell, the clone expands (key event 4), which can lead to an adverse outcome upon a second exposure to the chemical sensitizer. This level of mechanistic understanding has enabled the development of a series of non-animal test methods, each of which aims to measure the impact of substances on one of the AOP key events. These methods can distinguish sensitizers from non-sensitizers and assess potency.
Adverse Outcome Pathway and Predictive Testing
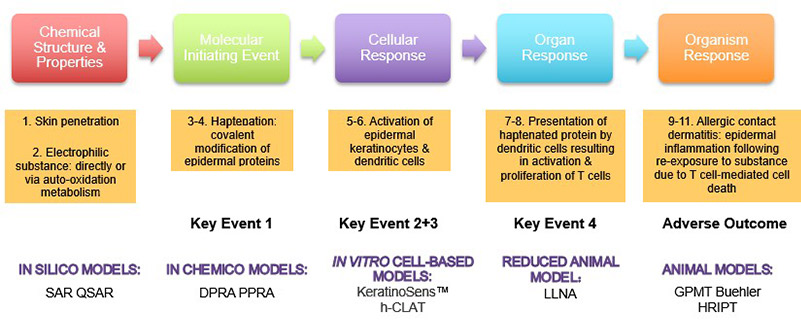
Our In Vitro Skin Sensitization Services
Three non-animal assays have been formally validated and adopted by regulators: the direct peptide reactivity assay (DPRA), the KeratinoSens™ assay, and the human cell line activation test (h-CLAT). Creative BioMart offers all three of these methods as part of our GLP-compliant laboratory testing services for non-animal skin sensitization testing.
|
Targeted Key Event |
Non-Animal Skin Sensitization Test Systems |
|---|---|
|
Key event 1: Covalent binding to proteins |
Direct Peptide Reactivity Assay (DPRA) In chemico method for predicting covalent protein binding. |
|
Key event 2: Keratinocyte inflammatory responses |
KeratinoSens™ Assay In vitro assay measuring keratinocyte activation via Nrf2-dependent gene signaling. |
|
Key event 3: Activation of dendritic cells |
Human Cell Line Activation Test (h-CLAT) In vitro dendritic cell activation assay using CD86/CD54 markers. |
Service Workflow

Advantages of Our Non-Animal Testing Expertise
- OECD-Validated Assays: Fully compliant with international regulatory standards.
- GLP-Certified Laboratory: Ensuring high data quality and regulatory acceptance.
- Comprehensive Coverage: Assays aligned with three major AOP key events.
- Advanced Technologies: HPLC, luminescence detection, and flow cytometry platforms.
- Regulatory Expertise: Data packages designed to meet FDA, EMA, and OECD guidelines.
- Flexible Solutions: Customized testing strategies integrated into your existing programs.
Case Studies: Non-Animal Skin Sensitization Applications
Case 1: Assessing skin sensitization hazard in mice and men using non-animal test methods
Urbisch et al., 2015. doi:10.1016/j.yrtph.2014.12.008
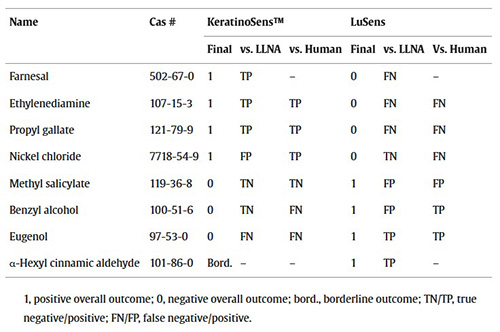
Sensitization, the initial step in allergic contact dermatitis, is a critical factor in hazard and risk assessments. The OECD adverse outcome pathway (AOP) for skin sensitization highlights that no single non-animal method can fully address the pathway, making test batteries essential. Validated methods such as DPRA, KeratinoSens™, LuSens, h-CLAT, and (m)MUSST have been evaluated individually and within integrated approaches like the “2 out of 3” model across 213 substances. These assays demonstrated strong predictivity against both animal and human data, with the “2 out of 3” model achieving up to 90% accuracy, surpassing even the LLNA in reliability.
Table 1. Substances with discordant test results among KeratinoSens™ and LuSens. (Urbisch et al., 2015)
Case 2: h-CLAT as a reliable in vitro alternative for skin sensitization testing
Sakaguchi et al., 2009. doi:10.1007/s10565-008-9059-9
With the European ban on animal testing in cosmetics, in vitro alternatives are urgently needed for assessing skin sensitization. This study focused on optimizing the human cell line activation test (h-CLAT) using THP-1 cells to measure CD86/CD54 expression as predictive markers. Twenty-one allergens and eight non-allergens were tested across multiple concentrations, showing distinct expression patterns. Most allergens required moderate cytotoxicity (65–90% viability) to enhance marker expression. Using criteria of CD86 ≥ 150 or CD54 ≥ 200 achieved 93% accuracy, with strong correlation to the local lymph node assay. These results confirm h-CLAT as a robust non-animal testing method.
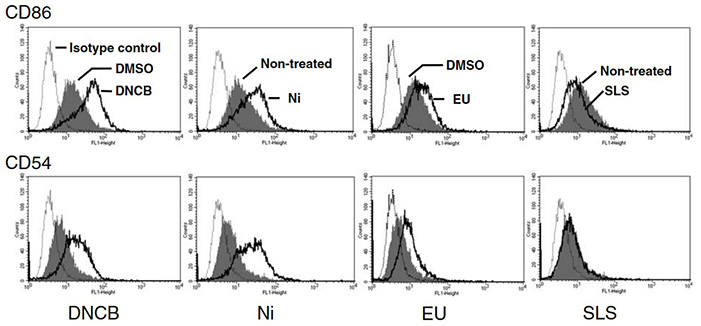
Figure 1. Histogram of CD86/CD54 expression after DNCB, Ni, EU, or SLS treatments. Alteration of CD86 and CD54 expression induced by DMSO (vehicle), DNCB (2.5 μg/ml), NiSO4 (85 μg/ml), EU (54 μg/ml), and SLS (58 μg/ml). Isotype control (dotted line), vehicle-control (either DMSO or untreated; shaded peak), and chemical-treated (dark solid line). MFI (Geometric) Mean fluorescence intensity. (Goebel et al., 2017)
Client Experiences with Our Testing Services
"As part of our safety evaluation for a new topical anti-inflammatory drug, we engaged Creative BioMart to conduct non-animal sensitization testing. Their team performed DPRA and h-CLAT assays, generating GLP-compliant data that aligned seamlessly with OECD requirements. The timely delivery and detailed mechanistic insights allowed us to refine our candidate before clinical submission, saving us months of potential delays."
— Director of Toxicology | Global Pharmaceutical Company
"We partnered with Creative BioMart to evaluate a series of new preservatives for use in skin care formulations. Their KeratinoSens™ and DPRA assays not only confirmed low sensitization potential but also provided potency data critical for regulatory dossiers in the EU market. Their transparent communication and regulatory expertise gave our R&D team great confidence throughout the process."
— Head of Safety Assessment | Leading Cosmetics Company
"For a novel insecticide compound under development, we required non-animal sensitization testing to meet EPA submission standards. Creative BioMart delivered a full testing package—including DPRA, KeratinoSens™, and h-CLAT—within tight timelines. Their ability to integrate findings into the Adverse Outcome Pathway framework provided clarity for both our regulatory and formulation teams."
— Senior Scientist, Regulatory Affairs | Multinational Agrochemical Company
"Creative BioMart supported us in assessing sensitization risks for an advanced wound care product. Their GLP-compliant KeratinoSens™ and h-CLAT assays were executed with precision, and the final report was structured in a way that could be directly incorporated into our ISO 10993 biological evaluation file. This significantly accelerated our device approval process in multiple markets."
— Director of Product Development | Medical Device Manufacturer
FAQs About Non-Animal Skin Sensitization Testing
-
Q: Why should I choose non-animal methods for skin sensitization testing?
A: Non-animal assays are not only more ethical but also regulatory-accepted alternatives that provide reliable mechanistic insights into skin sensitization. Our methods comply with OECD guidelines and are increasingly preferred by regulatory authorities worldwide, including the EU and US, reducing risks of rejection during submission. -
Q: What non-animal assays do you provide for skin sensitization testing?
A: We offer three validated and OECD-adopted assays:- DPRA (Direct Peptide Reactivity Assay) for covalent binding prediction (Key Event 1).
- KeratinoSens™ assay for keratinocyte activation (Key Event 2).
- h-CLAT (Human Cell Line Activation Test) for dendritic cell activation (Key Event 3).
-
Q: How do your services support regulatory submissions?
A: Our assays are performed in GLP-compliant laboratories and delivered with comprehensive reports that can be directly included in regulatory dossiers (e.g., OECD, EU REACH, EPA, ISO 10993). We also help design integrated testing strategies tailored to your submission requirements. -
Q: Can Creative BioMart help me decide which assays are appropriate for my compound?
A: Absolutely. Our scientists provide consultation on assay selection based on your compound’s properties, regulatory context, and intended application. This ensures cost-effective testing without compromising regulatory acceptance. -
Q: How quickly can I expect results?
A: Turnaround times vary depending on the assay package, but most projects are completed within 2–4 weeks. We also accommodate urgent timelines when needed. -
Q: What industries benefit most from your testing services?
A: Our non-animal skin sensitization assays support companies in cosmetics & personal care, pharmaceuticals, agrochemicals, and medical devices. Whether it’s a new skincare formulation, a novel drug, or a medical device, our testing services help ensure safety and compliance.
Resources
Related Services
References:
- Sakaguchi H, Ashikaga T, Miyazawa M, et al. The relationship between CD86/CD54 expression and THP-1 cell viability in an in vitro skin sensitization test – human cell line activation test (h-CLAT). Cell Biol Toxicol. 2009;25(2):109-126. doi:10.1007/s10565-008-9059-9
- Urbisch D, Mehling A, Guth K, et al. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regulatory Toxicology and Pharmacology. 2015;71(2):337-351. doi:10.1016/j.yrtph.2014.12.008
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

