Biosimilar Comparability Studies
The Role of Comparability Studies in Biosimilar Development
Biosimilar comparability studies are essential for demonstrating that a biosimilar product is highly similar to an approved reference biologic, despite minor differences in the manufacturing process. Unlike generic drugs, biosimilars are large, structurally complex molecules produced in living systems, making exact replication impossible.

The current regulatory guidelines for biosimilars permit a reduced drug development program, enabling faster market entry for biosimilar manufacturers. However, while the pathway to licensure is abbreviated compared to that of a novel biologic, biosimilars are not considered identical to their reference products. As a result, a series of rigorous evaluations is required to establish comparability in terms of quality, safety, and efficacy.
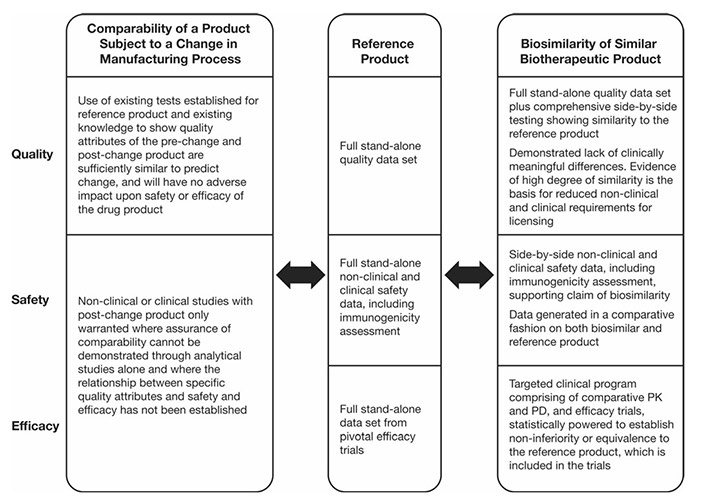
Figure 1. Regulatory guideline requirements on comparability and biosimilarity exercises. PD pharmacodynamics, PK pharmacokinetics. (Azevedo et al ., 2016)
Key attributes assessed include amino acid sequence, glycosylation profiles, aggregation behavior, and biological activity. Regulatory agencies such as the U.S. FDA, EMA, and other global authorities require a totality-of-evidence approach to confirm that no clinically meaningful differences exist between the biosimilar and its reference. These comparability studies ensure that biosimilars maintain the high standards expected of all biologic products, while facilitating broader access to safe, effective, and more affordable therapies.
As a world leader in biosimilar testing, Creative BioMart offers a comprehensive suite of advanced analytical assays designed to assess both the physicochemical and biological properties of biopharmaceutical products. Our services support the rigorous evaluation of biosimilarity by enabling detailed side-by-side comparisons between biosimilar candidates and their respective originator (reference) products.
Our Biosimilar Analytical and Functional Comparability Assessment Services
Service Procedure

Service Items
Creative BioMart provides a comprehensive range of assays to support biosimilar comparability studies, encompassing physicochemical characterization, structural analysis, and biological activity testing.
Physicochemical Property Analysis
-

Molecular Mass & Heterogeneity
- Ultracentrifugation
- SDS-PAGE
- Size-Exclusion HPLC (SE-HPLC)
- Laser Light Scattering
-
Isoform and Charge Variant Analysis
- Isoelectric Focusing (IEF)
- Capillary Electrophoresis
- Ion-Exchange HPLC (IE-HPLC)
-
Other Key Assessments
Extinction Coefficient Determination & Validation
Structural Characterization
-
Primary Structure
- Edman Degradation
- Peptide Mapping via LC-MS
- C-terminal Sequencing
- Amino Acid Composition Analysis
-
Secondary Structure
- Disulfide Bond Analysis using reduced/non-reduced peptide mapping with Edman degradation or MS
- Near-UV Circular Dichroism (CD)
-
Tertiary & Quaternary Structure
- Far-UV Circular Dichroism
- NMR Spectroscopy
- FTIR Spectroscopy
- X-ray Crystallography
-
Other Structural Features
- Free Thiol Group Determination
- Glycan Structure Analysis (Enzymatic cleavage + MALDI-MS, chromatography)
- Glycosylation Profiling / Fingerprinting
- Post-Translational Modification (PTM) Characterization
- Crystallographic Imaging via Microscopy
Biological Activity Testing
-

In Vivo Functional Assays
Evaluation of therapeutic effects in animal models
-
In Vitro Functional Assays
Cell-based assays for:
- Proliferation/Inhibition
- Senescence
- Cell Size / Morphology Changes
-
Mechanistic Bioassays
- Enzyme Activity Assays
- Receptor-Binding Assays
-
Coagulation Activity
Chromogenic and Turbidimetric Techniques to assess promotion or inhibition of coagulation pathways
Why Partner with Us for Biosimilar Comparability Studies
- Advanced Analytical Platforms : Equipped with state-of-the-art technologies—LC-MS, NMR, FTIR, CD, and more—for precise structural, functional, and PTM characterization.
- End-to-End Service Portfolio : From analytical characterization to in vivo studies, we provide complete comparability solutions under one roof for seamless project execution.
- Flexible Project Design : We offer fully customizable workflows—from cell models and assay formats to data outputs—to fit your specific scientific and regulatory objectives.
- Regulatory-Aligned Expertise : Our studies are designed to meet FDA, EMA, and ICH guidelines, supporting smooth submissions and confident regulatory interactions.
- Rapid Turnaround Times : With optimized workflows and in-house capabilities, we deliver high-quality data within accelerated timelines to keep your development on track.
- Scientific and Strategic Support : Our expert team collaborates with you throughout the project, offering scientific insight and technical guidance at every decision point.
Case Studies: Demonstrating Biosimilarity Through Robust Comparability
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Assessment of structural and functional comparability of trastuzumab biosimilar
Joshi and Rathore,2020. doi:10.1007/s40259-020-00404-3
Biotherapeutics are complex protein products whose quality is closely tied to their manufacturing process, making analytical comparability essential for biosimilar approval. This study evaluated the structural and functional comparability of four marketed trastuzumab biosimilars—Trasturel, Canmab, Vivitra, and Hertraz—against the originator Herclon. Using mass spectrometry, spectroscopy, thermal stability studies, and functional assays like ADCC and CDC, researchers found overall structural and functional similarity. However, one biosimilar showed significant deviations in monomer content, charge variants, glycoform composition, and potency. Despite general alignment, all biosimilars exhibited some variation in size, charge, and glycosylation profiles, underscoring the importance of thorough characterization.
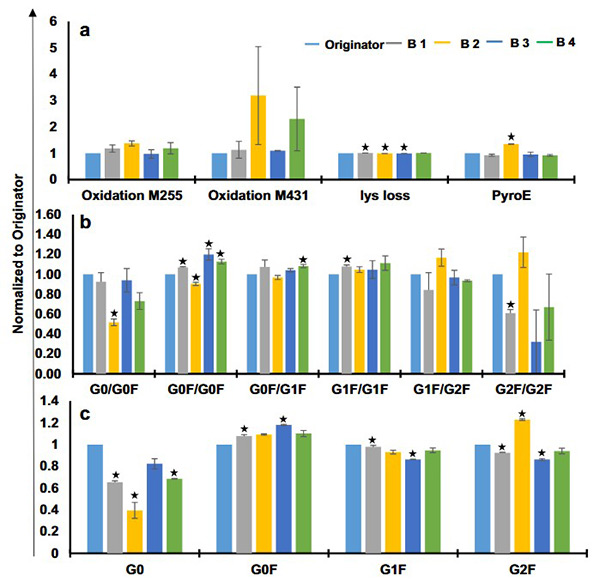
Figure 2. Glycoform analysis and posttranslational modifications of trastuzumab biosimilars and originator using liquid chromatography mass spectrometry. Relative quantification of a glycoforms for intact trastuzumab samples, b glycoforms for reduced trastuzumab samples, and c predicted posttranslational modifications for trastuzumab originator and biosimilars. (Joshi and Rathore, 2020)
Case 2: Evaluating omalizumab biosimilars with IgE-binding assays
Urbano et al . , 2023. doi:10.1016/j.biopha.2023.115848
Analytical and functional comparisons are crucial to determine the similarity between biologic drug variants. Using an orthogonal approach, researchers compared IgE-binding properties of various Xolair® (omalizumab) versions, analyzing binding kinetics via surface plasmon resonance and evaluating mast cell inhibition both in vitro and in vivo . Although monoclonal antibodies produced in CHO and HEK293 cells shared identical amino acid sequences, they showed slight differences in IgE binding and neutralization. A known variant with three Fab-region mutations had reduced binding affinity and neutralizing capacity. The applied assays proved sensitive enough to detect subtle Fab-linked differences, supporting biosimilar and new anti-IgE drug assessments.
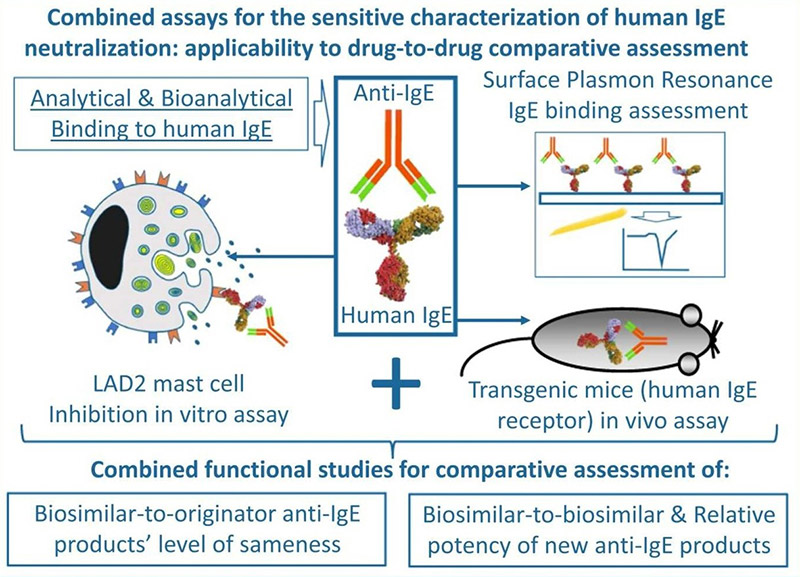
Figure 3. Graphic abstract of the evaluation of omalizumab biosimilars. (Urbano et al. , 2023)
What Our Clients Say About Our Biosimilar Support Services
"We partnered with Creative BioMart to evaluate the comparability of our biosimilar to adalimumab, focusing on glycosylation fingerprinting and receptor-binding affinity. Their team provided a detailed, stepwise study design that aligned perfectly with EMA guidelines. The level of technical expertise, coupled with fast turnaround, exceeded our expectations. Their data package was instrumental in supporting our regulatory submission."
— R&D Director | Mid-Sized European Biopharma Company
"As a small team developing a biosimilar version of a PEGylated G-CSF, we were concerned about demonstrating similarity in post-translational modifications and aggregation behavior. Creative BioMart’s comprehensive physicochemical and functional assays gave us full confidence in our molecule’s profile. Their use of SE-HPLC, peptide mapping, and in vitro cell proliferation assays was top-notch."
— Senior Scientist | U.S.-Based Biotech Startup
"What stood out was Creative BioMart’s regulatory insight. We needed head-to-head comparability data under ICH Q5E guidelines for our biosimilar erythropoietin. Their team not only delivered robust analytical and bioassay data, but also supported us during agency Q&A with clear, well-structured responses. The comparability report was submission-ready and highly professional."
— Regulatory Affairs Manager | Global Pharmaceutical Manufacturer
"Creative BioMart provided us with a full biosimilar testing package for our monoclonal antibody targeting VEGF. Their structural analysis—especially disulfide bond mapping via LC-MS and CD-based secondary structure evaluation—was extremely detailed. Excellent communication and scientific rigor throughout."
— VP of Preclinical Development | Asian Biologics CDMO
Frequently Asked Questions about Biosimilar Comparability
-
Q: Why are comparability studies necessary for biosimilars if the development pathway is reduced?
A: While the regulatory pathway for biosimilars is more streamlined than that of new biologics, comparability studies are still essential. Biosimilars are not identical to originator products due to the complexity of biologics manufacturing. Regulatory authorities require head-to-head comparisons to confirm similarity in quality, safety, and efficacy. These studies form the cornerstone of biosimilar approval. -
Q: Can you help us design a customized comparability study plan?
A: Absolutely. Our scientific team begins each project with a feasibility consultation to assess your molecule’s attributes, regulatory objectives, and development stage. We then design a fit-for-purpose, stepwise comparability strategy that meets both scientific and regulatory expectations, helping reduce time and cost without compromising data integrity. -
Q: What types of assays are included in your comparability service?
A: Our service includes:
- Physicochemical testing (e.g., SE-HPLC, capillary electrophoresis, mass spectrometry)
- Structural analysis (e.g., peptide mapping, CD, FTIR, NMR, glycosylation profiling)
- Functional assays (e.g., cell proliferation, enzyme activity, receptor binding)
- Optional in vivo studies to resolve residual uncertainties
-
Q: Do you support regulatory submissions?
A: Yes. We provide comprehensive data packages and comparability reports formatted for regulatory submission, including clear documentation of analytical similarity and scientific rationale for any observed differences. Our experts are available to support pre-submission meetings and respond to agency queries. -
Q: How long does a typical comparability study take?
A: Timelines depend on the molecule’s complexity and the extent of testing required. However, Creative BioMart is known for its efficient turnaround, with streamlined workflows that significantly reduce development time without sacrificing data quality. Project timelines are provided after initial consultation.
Resources
Related Services
Related Products
References:
- Azevedo V, Hassett B, Fonseca JE, et al . Differentiating biosimilarity and comparability in biotherapeutics. Clin Rheumatol . 2016;35(12):2877-2886. doi:10.1007/s10067-016-3427-2
- Joshi S, Rathore AS. Assessment of structural and functional comparability of biosimilar products: trastuzumab as a case study. BioDrugs . 2020;34(2):209-223. doi:10.1007/s40259-020-00404-3
- Urbano A, Plaza J, Picado C, De Mora F. Combined analytical assays for the characterization of drugs binding to human IgE: Applicability to omalizumab-bearing biosimilar candidates assessment. Biomedicine & Pharmacotherapy . 2023;169:115848. doi:10.1016/j.biopha.2023.115848
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

