Direct Peptide Reactivity Assay (DPRA)
The Direct Peptide Reactivity Assay (DPRA) is a validated in chemico method designed to assess the skin sensitization potential of chemicals by quantifying their reactivity toward synthetic peptides. As most skin sensitizers form covalent bonds with nucleophilic amino acid residues, DPRA provides a reliable mechanistic measure of a substance’s electrophilic reactivity, enabling early-stage hazard identification without the use of animal testing. At Creative BioMart, we deliver high-quality, regulatory-aligned DPRA studies supported by advanced analytical instrumentation, rigorous quality control, and fast turnaround times—empowering researchers and product developers to evaluate ingredient safety efficiently, ethically, and cost-effectively.
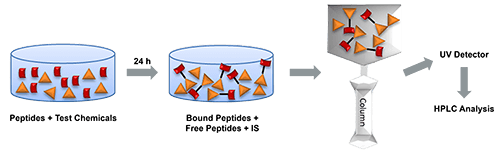
Introduction to Direct Peptide Reactivity Assay (DPRA)
Skin sensitization represents a key endpoint in toxicological evaluations across cosmetics, personal care products, agrichemicals, biocides, specialty chemicals, and pharmaceuticals. Traditional assessments have relied heavily on animal-based methods; however, both regulatory agencies and industry stakeholders increasingly emphasize non-animal approaches that provide mechanistic, reproducible, and ethically sound alternatives.
Figure 1. An optical fiber used for Bio-Layer Interferometry and a typical optical signal. (Nirschl et al., 2011)
The Direct Peptide Reactivity Assay (DPRA) is one such method, developed to model the first molecular initiating event in the skin sensitization adverse outcome pathway: covalent binding of electrophilic chemicals to skin proteins. Sensitizing chemicals generally exhibit electrophilic properties, allowing them to react with nucleophilic side chains such as the thiol group of cysteine or the amino group of lysine. DPRA uses synthetic heptapeptides containing either cysteine or lysine as reaction probes. DPRA quantifies the extent of peptide depletion—an indicator of chemical reactivity — by incubating a test chemical with these peptides under standardized conditions and measuring the remaining peptide concentrations after approximately 24 hours. Percent peptide depletion is calculated using the following formula:
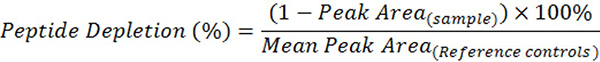
The assay has undergone rigorous validation by the European Center for the Validation of Alternative Methods (ECVAM) and has been endorsed as a reliable tool for preliminary hazard screening, weight-of-evidence approaches, and integrated testing strategies. It is now widely recognized by regulatory frameworks such as OECD Test Guideline 442C.
Direct Peptide Reactivity Assay (DPRA): Services & Capacities
Creative BioMart provides a comprehensive DPRA testing service designed to support safety assessment workflows for diverse chemical substances. Our service includes:
- Full execution of DPRA following ECVAM-validated and OECD-aligned protocols
- Cysteine 1:10 and lysine 1:50 peptide reactivity models
- High-performance HPLC-UV quantification of peptide depletion
- Accurate calculation of mean percent depletion and reactivity classification
- Detailed study reports including chromatograms, analytical interpretations, and sensitization predictions
- Support for regulatory submission, R&D screening, and formulation optimization
- Consultation on integrating DPRA data with other non-animal assays, such as KeratinoSens™ or h-CLAT, for a full skin-sensitization assessment strategy
Whether you are screening early-stage compounds, modifying formulations, or preparing regulatory dossiers, we provide a dependable, fast, and cost-efficient testing solution.
Service Workflow
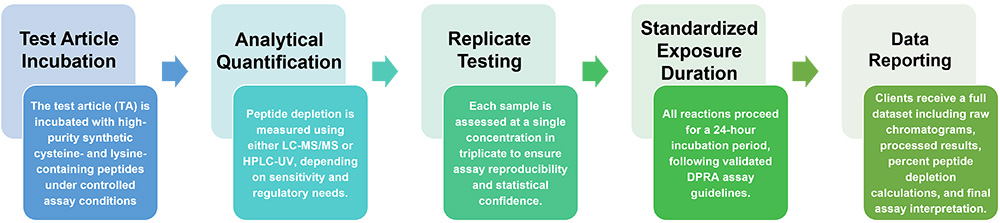
Results are interpreted according to recommended criteria:
Table 1. Recommended classification of reactivity class and sensitization potential based on the DPRA data (cysteine 1:10/lysine 1:50 model).
| Mean % depletion |
Reactivity class |
Prediction |
|---|---|---|
|
0–6.38% |
Minimal |
Non-sensitizer |
|
6.38%–22.62% |
Low |
Sensitizer |
|
22.62%–42.47% |
Moderate |
Sensitizer |
|
42.47%–100% |
High |
Sensitizer |
Service Features
-
Test Articles Accepted
We support evaluation of chemicals and ingredient libraries across numerous sectors, including:
- Cosmetics and personal care ingredients
- Agrochemical active compounds and intermediates
- Biocidal agents and preservatives
- Specialty chemical components
- Pharmaceutical small molecules or excipients
-
Assay Strengths and Scientific Basis
DPRA focuses on the molecular initiating event—protein binding—which is fundamental to the sensitization process. This mechanistic clarity supports DPRA’s use in early screening and integrated testing strategies. As electrophile–nucleophile interactions can often predict sensitization risk, DPRA offers a strong correlation with in vivo outcomes for many chemical classes.
-
Analytical Excellence
Creative BioMart employs:
- State-of-the-art HPLC instrumentation with validated detection wavelengths
- Automated sample handling to reduce variability
- Robust peptide stability checks before and after incubation
- Strict adherence to ECVAM validation recommendations
-
Regulatory Relevance
DPRA data contribute directly to:
- OECD-compliant test strategies
- REACH submissions
- Cosmetics Regulation (EC 1223/2009)
- GHS/CLP hazard classification workflows
What Sets Us Apart
- Fully Validated, Regulatory-Aligned Protocols: We follow the latest OECD 442C guidelines and ECVAM-endorsed procedures to ensure compliance with international regulatory requirements and scientific best practices.
- High Sensitivity and Reproducibility: Our optimized analytical platform ensures low background noise, stable peptide performance, and precise quantitation—leading to highly reproducible depletion values.
- Rapid Turnaround with Scalable Throughput: Whether you are testing a single substance or hundreds, our high-throughput setup ensures quick delivery without compromising quality.
- Cost-Efficient Screening for R&D and Formulation Workflows: DPRA is an economical way to prioritize candidates, modify formulations, and eliminate high-reactivity compounds early, reducing downstream development risks.
- Expert Scientific Support and Data Interpretation: Our specialists offer interpretation beyond numerical outputs, providing mechanistic insights and integration guidance with other skin sensitization assays.
- Commitment to Ethical and Non-Animal Testing: As part of our broader non-animal safety testing platform, we champion modern, humane approaches that support global regulatory trends and industry sustainability goals.
Direct Peptide Reactivity Assay (DPRA): Case Studies
Case 1: Using kDPRA to predict strong skin sensitizers
Natsch, 2020. doi:10.14573/altex.2004292
The kinetic Direct Peptide Reactivity Assay (kDPRA), an enhanced version of the OECD DPRA method, measures how quickly chemicals react with a cysteine-containing peptide—a key driver of skin sensitization. In a large dataset of 180 chemicals, researchers calculated log kmax values and identified a threshold of –2 that predicts strong sensitizers (GHS 1A) with about 85% accuracy. The assay showed strong reproducibility and can function as a stand-alone tool for identifying high-potency sensitizers or be integrated with other in vitro data for continuous potency assessment. This positions kDPRA as a practical, quantitative approach for refining sensitization hazard evaluation.
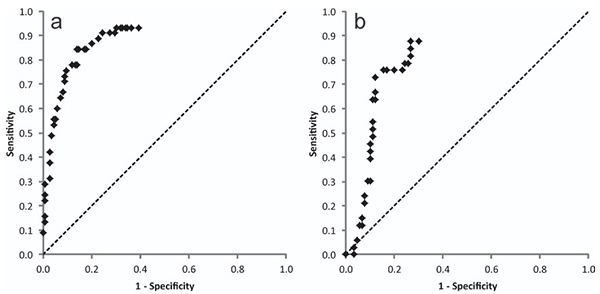
Figure 2. ROC curves for different log k max threshold values to discriminate GHS Cat 1A from 1B/NC chemicals as determined by LLNA data (a) and human data (b). (Natsch, 2020)
Case 2: Advancing DPRA with high-throughput SPE-MS/MS
Wei et al., 2021. doi:10.1016/j.toxlet.2020.12.002
Researchers developed an automated 384-well RapidFire SPE-MS/MS version of the Direct Peptide Reactivity Assay (DPRA) to improve detection of chemical–peptide conjugation—the key molecular trigger of skin sensitization. This upgraded method dramatically increased throughput from 16 minutes to 10 seconds per sample and reduced peptide consumption from 100 mM to 5 μM. In testing 55 chemicals, it identified multiple lysine- and cysteine-reactive sensitizers, including previously unreported ones. Additional HPLC-TOF-MS analysis confirmed specific peptide adducts. Overall, this high-throughput SPE-MS/MS DPRA provides a faster, more economical, and highly accurate approach for sensitizer screening.
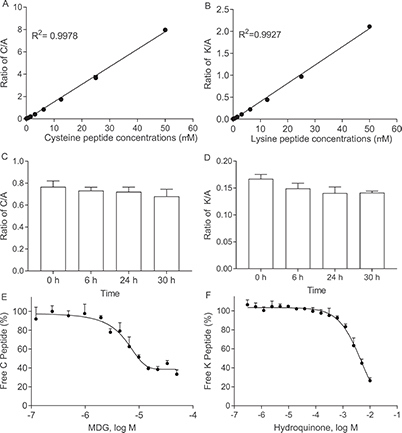
Figure 3. Validation of the cysteine and lysine peptide depletion assays. Linear response of cysteine (A) and lysine (B) peptide calibration standards detected by the RapidFire-MS/MS system. Stability of cysteine (C) / lysine (D) peptide mixed alanine peptide up to 30 h. Concentration response curves of (E) MDG- and (F) hydroquinone-induced cysteine/lysine peptide depletion. (Wei et al., 2021)
Direct Peptide Reactivity Assay (DPRA): Customer Feedback
"Our toxicology group relied on Creative BioMart to perform DPRA screening on a library of 80 new cosmetic fragrance candidates. Their ability to process the entire panel quickly using an ECVAM-validated workflow was invaluable. The peptide-depletion data was clear, well-annotated, and ready for immediate integration into our internal safety assessment system. Their early identification of several high-reactivity compounds saved us weeks of downstream formulation work."
— Senior Toxicology Manager | Global Personal Care Company
"We needed rapid sensitization profiling for a set of agrochemical intermediates undergoing regulatory review. Creative BioMart delivered DPRA results ahead of schedule and with a level of analytical rigor that exceeded our expectations. Their team provided detailed LC-MS/MS chromatograms and walked us through the interpretation of borderline reactivity values. This clarity helped us refine our structure–activity relationship models with confidence."
— Director of Chemical Safety Evaluation | Multinational Agrichemical Corporation
"Our early-stage drug discovery team partnered with Creative BioMart to evaluate a group of small-molecule kinase inhibitors for skin sensitization risk. Their DPRA service proved incredibly efficient—the triplicate testing, controlled exposure conditions, and comprehensive reporting allowed us to progress only the safest candidates into in vitro follow-up assays. The communication throughout the project was excellent, especially when discussing peptides showing unexpected depletion patterns."
— Head of Preclinical Development | Innovative Biopharmaceutical Company
"Creative BioMart supported our formulation R&D group by conducting DPRA testing on several prototype preservative blends. Their cost-effective assay setup and rapid turnaround enabled us to iterate quickly and optimize the compositions. The side-by-side comparison of cysteine and lysine depletion data was particularly helpful for identifying which components were driving reactivity. Their expertise significantly streamlined our ingredient selection process."
— R&D Formulation Director | International Household Products Manufacturer
Direct Peptide Reactivity Assay (DPRA): Frequently Asked Questions (FAQs)
-
Q: What types of substances can be evaluated using DPRA?
A: DPRA is suitable for a wide range of chemicals, including cosmetic ingredients, pharmaceuticals, biocides, agricultural formulations, specialty chemicals, and industrial intermediates. Because the assay evaluates electrophilic reactivity with nucleophilic peptides, it is particularly useful during early screening and pre-formulation stages across multiple industries. -
Q: How reliable is the DPRA method for predicting skin sensitization?
A: DPRA is an ECVAM-validated and internationally recognized assay that provides high-confidence early-stage sensitization predictions. It is widely accepted as part of weight-of-evidence strategies and integrated testing approaches for regulatory submissions. Our use of validated procedures, synthetic peptides of consistent quality, and strict analytical controls ensures accurate and reproducible data. -
Q: What analytical platforms do you use, and how does this benefit my project?
A: We offer both LC-MS/MS and HPLC-UV platforms. HPLC-UV provides a robust, cost-efficient option for routine screening. LC-MS/MS offers higher specificity and sensitivity, especially valuable for complex matrices or compounds with similar UV absorbance. Customers can choose the most suitable analytical mode based on project needs, budget, and regulatory expectations. -
Q: Can DPRA accommodate complex or poorly soluble test substances?
A: Yes. Our team has extensive experience optimizing solubility conditions, including the use of appropriate solvents, co-solvents, or diluents that remain compatible with peptide stability. We provide recommendations during project initialization to ensure that poorly soluble or reactive materials are evaluated reliably. -
Q: How long does the DPRA workflow take from sample submission to data delivery?
A: The assay itself involves a single 24-hour exposure followed by data acquisition on LC-MS/MS or HPLC-UV. Most projects are completed within 5–7 business days depending on sample number and analytical platform. Accelerated timelines can also be arranged for urgent screening needs. -
Q: What information will I receive in the final report?
A: You will receive:- Raw chromatographic data and processed results
- Percent depletion for cysteine and lysine peptides
- Mean peptide depletion and calculated reactivity class
- Sensitization prediction (minimal, low, moderate, or high reactivity)
- Method summary, controls validation, and QC metrics
-
Q: Can you test multiple concentrations or perform extended exposure conditions?
A: While standard DPRA uses a single concentration in triplicate and a fixed 24-hour exposure, we can customize the design upon request. For research or mechanistic studies, we can evaluate additional concentrations or timepoints to provide more nuanced reactivity profiles.
Other Resources
Related Services
References:
- Natsch A. Predictivity of the kinetic direct peptide reactivity assay (kDPRA) for sensitizer potency assessment and subclassification. ALTEX. Published online 2020. doi:10.14573/altex.2004292
- Wei Z, Fang Y, Gosztyla ML, et al. A direct peptide reactivity assay using a high-throughput mass spectrometry screening platform for detection of skin sensitizers. Toxicology Letters. 2021;338:67-77. doi:10.1016/j.toxlet.2020.12.002
- Wong CL, Ghassabian S, Smith MT, Lam AL. In vitro methods for hazard assessment of industrial chemicals – opportunities and challenges. Front Pharmacol. 2015;6. doi:10.3389/fphar.2015.00094
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

