Rice Endosperm Specific Expression Platform
Creative BioMart offers a cutting-edge Rice Endosperm Specific Expression Platform as part of our Animal Component-Free Process (ACFP) services. By leveraging rice endosperm cells as bioreactors, we provide a safe, scalable, and cost-effective solution for producing high-quality recombinant proteins and peptides. This platform delivers proteins with high expression levels, bioactivity, and purity—ideal for therapeutic, industrial, and cosmetic applications. With customized service offerings, rigorous SOPs, and environmentally friendly production, we support partners from early-stage R&D to commercial-scale manufacturing.

Overview of Rice Endosperm Expression Platform for Protein Production
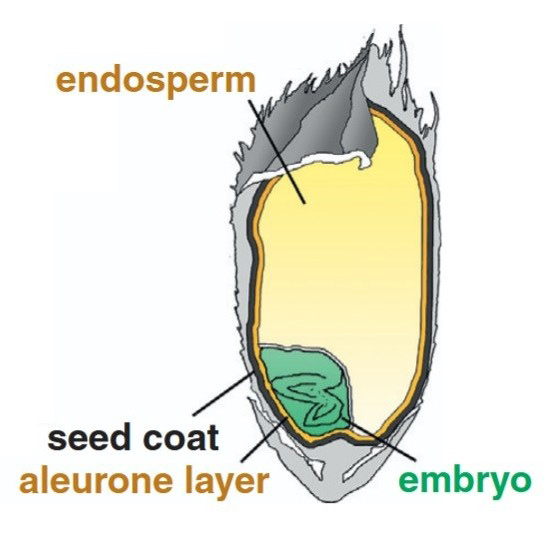
Figure 1. Endosperm of rice. (Adapted from Zhou et al., 2013)
The rice endosperm is the primary storage tissue in rice seeds, naturally equipped to accumulate large amounts of proteins. It offers a stable and high-yield platform for heterologous protein expression, especially valuable for producing therapeutic proteins, nutraceuticals, and industrial enzymes.
Unlike microbial or mammalian systems, plant-based expression systems like rice are inherently safer—free from mammalian pathogens, toxins, and allergens. Rice, as a staple crop, is globally cultivated, well-studied, and has demonstrated successful expression of clinically relevant proteins, even achieving molecular identity with approved plasma-derived drugs.
Creative BioMart’s rice endosperm system merges decades of expertise in protein engineering with the biological advantages of plants, offering an animal-free, sustainable, and robust alternative to conventional protein expression platforms.
Service Overview: Rice Endosperm Expression Platform for Custom Protein Production
Service Procedure
|
Step 1 |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
|
Consultation & Design |
Vector Construction & Transformation |
Protein Expression & Accumulation |
Purification & Quality Analysis |
Delivery & Support |
|
|
|
|
|
Service Details
We provide both custom protein expression and pilot studies for a wide range of biomolecules:
- Therapeutic proteins & antibodies
- Human nutrition & health supplements
- Industrial enzymes
- Cosmetic and skincare actives
- Cell culture media supplements
- Small peptides with medical or nutritional uses
Our expression system also supports complex post-translational modifications and ensures the high bioactivity and safety required for pharmaceutical and clinical research applications.
Benefits of Our Rice Endosperm Protein Expression Services
- Animal-Free & Pathogen-Free : Our plant-based system eliminates the risk of mammalian pathogen contamination, making it ideal for biopharmaceuticals and clinical-grade products.
- High-Yield & Scalable : This platform delivers high expression levels with stable accumulation in rice seeds, supporting cost-effective scale-up through agricultural cultivation.
- Broad Application Range : From therapeutic proteins and peptides to cosmetics and food-grade enzymes, our platform supports diverse applications with consistent quality.
- Simplified Purification : Endosperm-specific expression enables tissue-targeted protein accumulation, simplifying downstream processing and reducing purification costs.
- Environmentally Sustainable : Rice-based production requires fewer resources than microbial or mammalian systems—offering an energy-efficient and eco-conscious alternative.
- Proven Bioactivity & Stability : Proteins produced retain their structure and function, with demonstrated biological activity, long shelf life, and transportability—even without cold chain logistics.
Rice Endosperm Platform Applications in Protein Manufacturing
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Optimized recombinant protein yield and extraction via endosperm-specific expression and SSP suppression
Takaiwa et al., 2021. doi:10.1016/j.plantsci.2020.110692
Transgenic rice seeds were engineered to produce human transforming growth factor-β1 (hTGF-β1) at high levels by using seed-specific promoters and suppressing endogenous seed storage proteins (SSPs) via RNA interference. The 26 kDa α-globulin promoter enabled the highest yield at 452 μg/grain, while other promoters like 18 kDa oleosin and AGPase led to significantly lower yields (62 and 48 μg/grain). Suppression of SSPs improved solubility during extraction—hTGF-β1 could be efficiently solubilized with denaturant alone, unlike seeds with intact SSPs that required reducing agents. The improvement is linked to hTGF-β1 localization in ER-derived protein bodies and reduced disulfide-mediated aggregation.
Figure 2. Expression of hTGF-β1 and effect on expression of SSPs during seed maturation of transgenic rice harbouring the Glbpro:hTGF-β1+RNAi or Ubipro:hTGF-β1 construct. Effect of production of TGF-β1 on expressions of seed proteins, transcription factors involved in SSP expression and ER chaperones was investigated by immunoblot analysis. (Takaiwa et al., 2021)
Case 2: Enhanced Immunogenicity of Rice-Derived CSFV E2 via Herringbone Dimer Design
Xu et al., 2023. doi:10.1111/pbi.14152
Researchers developed a novel vaccine strategy using a herringbone-dimer design to enhance immune response against classical swine fever virus (CSFV), a major threat to global livestock. The E2 antigen from CSFV was successfully expressed in rice endosperm, reaching yields of 480 mg/kg. Animal studies in mice, rabbits, and pigs confirmed the strong antigenicity of the recombinant E2 (rE2) protein. Just two doses of 284 ng or a single 5.12 μg shot protected pigs effectively, even in the presence of pre-existing antibodies. Structural analyses confirmed a stable, immune-accessible dimeric form. This approach highlights rice endosperm’s potential for scalable, precise vaccine development for Flaviviridae viruses.
Figure 3. Antibody blocking rates in pigs without (Light) and with (Right) maternal antibodies after immunization with varying rE2 doses (284 ng to 5.12 μg), compared to live attenuated vaccine. (Xu et al., 2023)
Client Feedback on Our Rice Endosperm Expression Platform
“We were looking for an alternative to CHO cells to express a bioactive cytokine for topical skin care. Creative BioMart’s rice endosperm system delivered surprisingly high expression levels with excellent protein stability and purity. The animal-free process also aligned perfectly with our vegan product line. This partnership allowed us to speed up formulation work and meet clean-label claims ahead of schedule.”
— Senior Scientist | Natural Cosmetics Startup
“Our team needed to produce a recombinant enzyme for a rare metabolic disorder with stringent safety requirements. Creative BioMart’s rice endosperm platform offered a contamination-free, scalable option that checked all the regulatory boxes. They helped us achieve >90% purity with low endotoxin levels and a consistent glycosylation profile. The ability to scale up through seed propagation was a huge plus for cost control.”
— Director of Bioprocess Development | Rare Disease Biotech
“We engaged Creative BioMart for the expression of a small peptide hormone that was difficult to produce in bacterial systems due to aggregation. Their rice endosperm platform yielded stable expression and allowed us to isolate a functional peptide without the need for extensive refolding steps. Their downstream team worked closely with us to develop a simple purification workflow that cut our COGs by 30%.”
— R&D Lead | Peptide Therapeutics Company
“Our nutritional supplement company was seeking a sustainable way to produce recombinant lactoferrin without animal-derived inputs. Creative BioMart’s rice-based platform exceeded our expectations. The final product was free of contaminants and matched the activity profile of bovine-sourced versions.”
— Formulation Manager | Functional Food Manufacturer
FAQs–Rice Endosperm Protein Expression Service
-
Q: What types of proteins or peptides can be expressed using your rice endosperm platform?
A: Our platform supports a wide range of target molecules, including therapeutic proteins, antibodies, enzymes, small peptides, and even bioactive compounds for cosmetics, nutrition, and cell culture media. Custom projects are welcomed. -
Q: Is your rice endosperm expression system animal component-free?
A: Yes, our entire production process is conducted under strict Animal Component-Free Process (ACFP) guidelines — from raw materials and labware to media and purification—ensuring safety, compliance, and clean-label compatibility. -
Q: How does rice endosperm expression compare to traditional systems like CHO or E. coli ?
A: Our rice system offers cost-efficiency, low contamination risk, and scalable production. Unlike microbial systems, it supports post-translational modifications and avoids endotoxins or mammalian pathogens — ideal for pharmaceutical and nutritional applications. -
Q: What are the typical expression levels achievable in the rice endosperm system?
A: Expression yields can vary by protein but are typically high due to the natural storage properties of the rice endosperm. We’ve achieved up to gram-per-kilogram levels for some targets with high bioactivity and purity. -
Q: Do you offer customization or pilot studies before full-scale production?
A: Absolutely. We specialize in tailored solutions, from initial gene construct design to pilot studies and troubleshooting protein purification challenges. We collaborate closely with clients to ensure optimal results. -
Q: Is the rice endosperm system suitable for GMP production?
A: Yes, the platform is compatible with cGMP manufacturing. We follow stringent SOPs, and the system’s natural containment, consistency, and low bioburden make it ideal for regulated environments.
Resources
Related Services
- Mammalian Expression Systems
- Yeast Expression Systems
- Baculovirus-Insect Cell Expression
- Bacterial Expression Systems (E. coli / Bacillus)
- Nicotiana tabacum Specific Expression Platform
- Cell-Free In Vitro Protein Expression
- Serum-Free Expression System
- Protein Expression and Purification Services
- Codon Optimization
Related Products
References:
- Takaiwa F, Wakasa Y, Ozawa K, Sekikawa K. Improvement of production yield and extraction efficacy of recombinant protein by high endosperm-specific expression along with simultaneous suppression of major seed storage proteins. Plant Science. 2021;302:110692. doi:10.1016/j.plantsci.2020.110692
- Xu Q, Ma F, Yang D, et al. Rice‐produced classical swine fever virus glycoprotein E2 with herringbone‐dimer design to enhance immune responses. Plant Biotechnology Journal. 2023;21(12):2546-2559. doi:10.1111/pbi.14152
- Zhou SR, Yin LL, Xue HW. Functional genomics-based understanding of rice endosperm development. Current Opinion in Plant Biology. 2013;16(2):236-246. doi:10.1016/j.pbi.2013.03.001
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

