Monkeypox Virus-related Proteins
Background
Since the outbreak of COVID19, our attention to the epidemic has been increasingly drawn. The prevailing view is that our understanding of monkeypox is not as ignorant as in the early stage of SARS-CoV-2, which strongly advanced our studies in overcoming this obstacle. In addition, smallpox vaccines have demonstrated approximately 85% protection effectiveness against the infection of monkeypox viruses, which is far lower than coronavirus in terms of transmission.
Monkeypox, a "close relative" of smallpox that has left terrible memories on humankind, was recently diagnosed with over 100 cases in the non-endemic areas outside Central and West Africa. As of May 21, the World Health Organization (WHO) has received a total of 92 laboratory-confirmed cases and 28 suspected cases of monkeypox under investigation in 12 countries and regions, including the United Kingdom, United States, Portugal, Spain, Italy, and Sweden. All cases have been found to include young males predominantly, with no associated deaths reported yet.
Monkeypox virus belongs to the genus Orthopoxvirus of the Poxviridae family and is a zoonotic infectious disease. Since the global eradication of smallpox in 1980, monkeypox has become a major epidemic of human orthopoxvirus infection. Infection with the monkeypox virus is often accompanied by clinical features such as fever, severe headache, swollen lymph nodes, myalgia, and weakness, among which lymphatic symptoms are a distinguishing feature of monkeypox.
Specifically, monkeypox viruses currently include two recognized clades – the West African strain and the Congo Basin strain. The epidemiological and clinical characteristics of diseases caused by these two clades of viruses are different, with a case fatality rate as high as 10% for the Congo Basin strain and a lower case fatality rate of about 1% for the West African strain. However, a report released by the European Centre for Disease Prevention and Control has elevated the West African strain's case fatality rate to 3.6%.
In 2018, the WHO included monkeypox in the priority list of diseases to control, that is, a rapid assessment of monkeypox is needed to formulate countermeasures. Moreover, it is also mentioned that smallpox vaccines and related treatment methods provide good references for the treatment and prevention of monkeypox.
The transmission route of the virus has an important impact on the spreading and growth of the epidemic. As a zoonotic disease, monkeypox was transmitted to humans through direct contact with the blood, body fluids, lesions of skins, or mucous membranes of infected animals. Human-to-human transmission of monkeypox is caused by close contact with respiratory secretions, skin lesions, or recently contaminated objects of the infected person.
Currently, there is no dedicated monkeypox vaccine, but the WHO has revealed data indicating that smallpox vaccines are 85% effective against monkeypox viruses. Moreover, the United Kingdom has started offering smallpox vaccines to healthcare workers or personnel at risk of monkeypox viruses, with mainly symptomatic and supportive clinical observations. (The US FDA approved Siga Technologies' Tecovirimat capsules for the treatment of smallpox on July 13, 2018. Tecovirimat targets the orthopoxvirus VP37 protein and inhibits its activity, blocking its interaction with cellular GTPase and TIP47.)
In order to provide better services for scientific researchers, Creative BioMart designed proteins according to the gene sequence of Monkeypox viruses. We have successfully developed various recombinant proteins related to Monkeypox viruses, and you're more than welcome to contact us for more information.
Case Study
Case 1: Li Y, Hou J, Sun Z, Hu J, Thilakavathy K, Wang Y, Shao Z, Lu Y, Wang W, Xiong C. Monkeypox virus 2022, gene heterogeneity and protein polymorphism. Signal Transduct Target Ther. 2023 Jul 17;8(1):278. doi: 10.1038/s41392-023-01540-2. PMID: 37460567; PMCID: PMC10352349.
Human monkeypox (MPX), an endemic disease in equatorial Africa,1 has been causing outbreaks in non-endemic countries since early May 2022. The researchers have analyzed 27 genes or sequences from 643 full-length human monkeypox virus (MPXV) genomes. They divided these 643 genomes into 24 clusters with a 95% identity threshold. The phylogenetic trees of the 26 genes or sequences (D14L was not detected in any MPXVs 2022) can be categorized into four types.
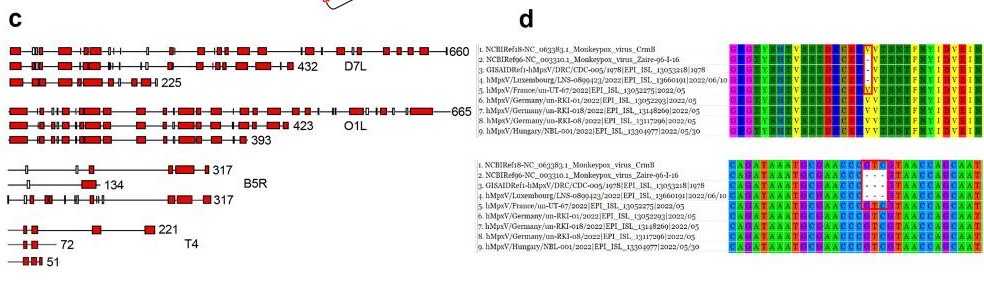
Fig1. Gene heterogeneity and protein polymorphism of Monkeypox viruses in 2022. c The proteins encoded by D7L, O1L, B5R, and T4 genes of MPXVs. In each of them, the first is the standard length of the protein, while the others are its variants. Proteins are shown as α-helix domains (according to the Gamier-Robson method) here. d The crmB gene (below) and its encoding protein (upper) of hMpxV/Luxembourg/LNS-0899423/2022. This strain is from the West African clade but displays an interesting hallmark belonging to the Congo Basin clade.
Case 2: Venturi C, Guadagno A, Varesano S, Ricucci V, Nigro N, Di Biagio A, Bassetti M, Nozza P, Drago F, Parodi A, Ciccarese G. Histopathologic and transmission electron microscopic findings in monkeypox cutaneous lesions. Infez Med. 2024 Mar 1;32(1):76-82. doi: 10.53854/liim-3201-10. PMID: 38456031; PMCID: PMC10917568.
A few pathologic and ultrastructural findings of monkeypox skin lesions are available in the literature. To integrate such evidence, the authors aimed to describe the pathologic features of monkeypox skin lesions and to show monkeypox virions by transmission electron microscopy (TEM). In all the samples the epidermis showed keratinocytes ballooning degeneration; perivascular/periadnexal infiltrates composed of neutrophils and lymphocytes were observed in the deep dermis. Immunohistochemistry showed that the infiltrate was mostly composed of CD3+ T-cells. TEM revealed monkeypox virus-like particles in various stages of morphogenesis in the dermis and epidermis; virions were interspersed among keratinocytes and within their cytoplasm.
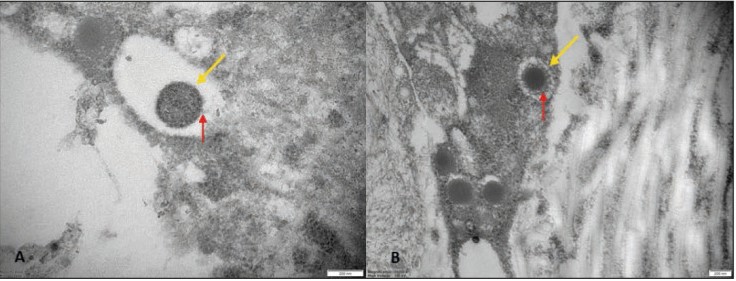
Fig1. Transmission electron microscopy. Epidermal-dermal extracellular environment: viral particles with an electrondense core (yellow arrow) and a surface characterized by inhomogeneously (A, red arrow) or evenly distributed protrusions “threads” (B, red arrow) near the collagen fibers (magnification: 50000× [A], 30000× [B]).
Case 3: Peng Q, Xie Y, Kuai L, Wang H, Qi J, Gao GF, Shi Y. Structure of monkeypox virus DNA polymerase holoenzyme. Science. 2023 Jan 6;379(6627):100-105. doi: 10.1126/science.ade6360. Epub 2022 Dec 15. PMID: 36520947.
The World Health Organization declared mpox (or monkeypox) a public health emergency of international concern in July 2022, and prophylactic and therapeutic measures are in urgent need. The monkeypox virus (MPXV) has its own DNA polymerase F8, together with the processive cofactors A22 and E4, constituting the polymerase holoenzyme for genome replication. Here, the authors determined the holoenzyme structure in complex with DNA using cryo-electron microscopy at the global resolution of ~2.8 angstroms.
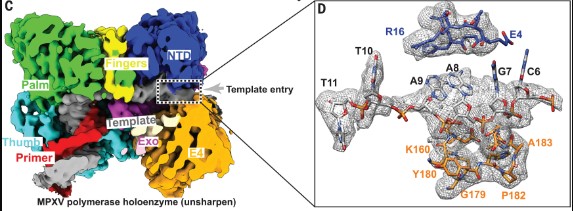
Fig1. The interactions between DNA and MPXV polymerase. C) The cut-off view of unsharpened EM density map for MPXV polymerase holoenzyme in complex with the DNA, revealing the consecutive density of template DNA. (D) Enlarged view of the unsharpened densities and supposed atomic models of the template DNA in the template entry channel. The region (C6 to T11) was built using this unsharpened map. The remaining 5′-terminal five bases of template DNA were invisible, which reflects an inherent flexible conformation of the 5′-terminal unpaired region of the template strand.
Application
As of now, research into the applications of Monkeypox Virus-related proteins is still relatively early-stage. However, they have shown potential utility in several areas:
Vaccine Development: Understanding the structure and function of Monkeypox virus-related proteins can aid in the development of potential vaccines against the virus.
Antiviral Drug Discovery: As in the case of HIV, Hepatitis C, and other illnesses, understanding the key proteins that allow Monkeypox to replicate and infect hosts can guide the development of antiviral drugs.
Diagnostic Tools: Identifying and understanding specific proteins associated with Monkeypox infection could aid the development of improved diagnostic tests.
Understanding Viral Pathology: Studying proteins related to Monkeypox (and viruses more broadly) allows scientists to better understand how these pathogens function, how they cause disease, and how the immune system responds to them.
Biological Research: Proteins from pathogens like Monkeypox can be used as biological research tools, helping scientists to probe the workings of cells and to understand biological processes.
However, this area of science is very complex, and the successful application of such proteins is not guaranteed. For example, while a protein may appear to be a suitable target for a drug, it may not be possible to design a safe and effective drug that interacts with this protein in the desired way. Nevertheless, research into Monkeypox and related viruses continues to be an active and important field of study.
















