Immunoprecipitation
Principle
Crosslink immunoprecipitation refers to the process of antigen purification utilising a specific antibody. The principle involves crosslinking to covalently attach a specific antibody to a protein A agarose resin (recombinant protein combining antibody-binding domains from protein A), which is then incubated with a protein mixture containing the antigen, allowing the antibody/antigen complex to form. After washing to remove unbound, undesired sample components, the antibody/antigen complex immobilised by crosslinking with disuccinmidyl suberate (DSS) on beaded agarose resin, is eluted and separated by centrifugation (see Figure 1). Samples under investigation were immunoprecipitated utilising a Pierce Crosslink IP kit, with all steps carried out at 4°C (unless otherwise stated) and all centrifugations at low speed (1000-3000 g) for 30-60 seconds.
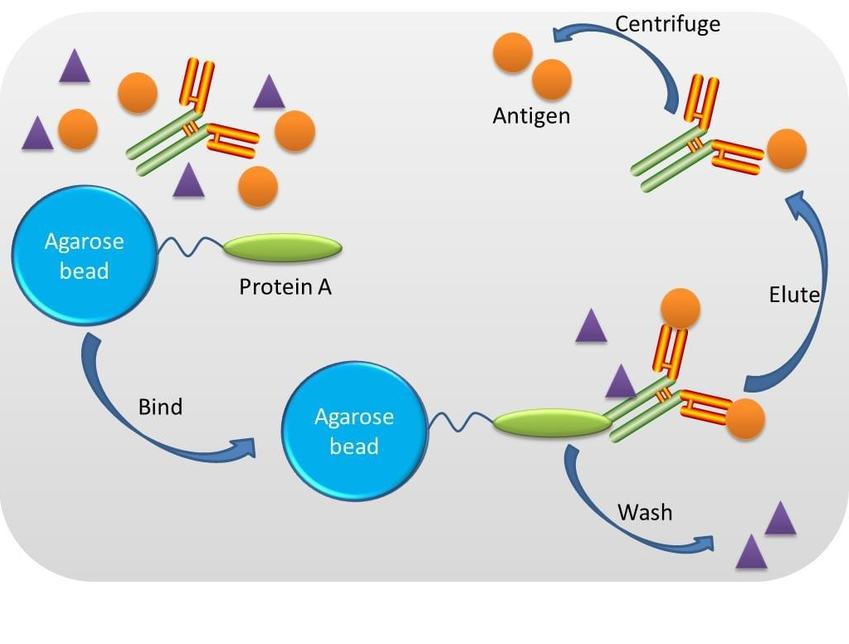
Fig. A schematic diagram of crosslink immunoprecipitation.
Procedure
Binding of antibody with Protein A Agarose Resin
- A solution containing 10 µg of antibody is prepared through dilution of the antibody with coupling buffer and ultrapure water.
- Then add 20 µl of Pierce Protein A/G Plus Agarose resin.
- Mix on a rotor for 30-60 minutes.
- Wash via centrifugation with coupling buffer.
Crosslinking of bound antibody
- Resin complex is incubated with DSS, ultrapure water and coupling buffer and placed on a mixer for 30-60 minutes at room temperature.
- The solution is washed in Elution buffer and IP / Lysis Wash buffer via centrifugation, prior to proceeding to immunoprecipitation.
Immunoprecipitation
- The sample under investigation is diluted with IP / Lysis Wash buffer to create a volume of 300-600 µl containing 500-1000 µg of total protein.
- The solution above is added to the prepared antibody resin complex and incubates overnight on a rotor at 4 °C.
Antigen Elution
- The mixture is washed via centrifugation with IP / Lysis Wash buffer.
- Conditioning buffer and 5 ul of 1 M Tris (pH 9.5) are added to neutralize the low pH and allow functional assays.
- The sample is incubated and centrifuged with elution buffer and the eluate collected for further analysis or stored at -20 °C.

