Protein Quantitation Service
With decades of expertise in protein research, Creative BioMart offers comprehensive and reliable Protein Quantitation Services to support every stage of biopharmaceutical development. From early discovery to late-stage clinical trials and commercialization, our services provide high-quality, accurate, and reproducible protein quantitation data. Whether you require assay transfer, development, verification, qualification, or full validation, our team is equipped to deliver results under R&D, GLP, or GMP conditions. With advanced technologies, strict quality control, and professional project management, we help clients achieve regulatory compliance and scientific confidence.

Understanding Protein Quantitation and Its Importance
Protein quantitation plays a pivotal role in modern biological and pharmaceutical research. Accurate measurement of protein concentration, activity, and expression levels is essential for understanding molecular pathways, assessing therapeutic efficacy, and meeting stringent regulatory requirements. Traditional methods such as spectrophotometry have long been used for protein measurement, but the growing complexity of research demands more advanced, high-throughput, and highly sensitive technologies.
At Creative BioMart, we provide an integrated portfolio of protein quantitation methods—from classic colorimetric assays to cutting-edge LC-MS/MS multiplex analysis—designed to accommodate diverse research needs and project scales.
Protein Quantitation Services & Capabilities
Our comprehensive protein quantitation services include:
Active Concentration Analysis
-
Enzyme Activity Assays (kinase, esterase, phosphatase, dehydrogenase, synthetase, etc.)
-
MMP and Adhesion Panel Analysis
-
Transcription Factor Assays (e.g., NF-кB P65)
-
Cytokine, Chemokine, and Growth Factor Quantitation
-
Promoter Methylation Array Studies
-
ATP Determination Assays
-
Antioxidant/Free Radical Testing
-
Biochemical Metabolism Assays
-
Tumor Marker and Disease Detection
Standard Concentration Measurement
- UV/visible spectrophotometry (Ultraspec series and related platforms)
- Classic protein quantitation methods: Bradford, BCA, Biuret, Lowry
- Absorbance, transmittance, OD600, and concentration-based determinations
iTRAQ Multiplex Protein Quantitation
- LC-MS/MS–based multiplex tagging with iTRAQ or dimethyl reagents
- Differential protein expression profiling
- Advanced peptide separation using SCX or HILIC chromatography
- Database searching and peptide identification with QTOF or Orbitrap MS
Broad Coverage of Protein Categories
Our quantitation services cover a wide spectrum of protein classes, enabling comprehensive analysis across diverse biological pathways and disease areas.
|
Categories |
Proteins |
|---|---|
|
CCL21/6Ckine , CCL2/MCP-1 , EGF , CXCL5/ENA-78 , CCL11/Eotaxin-1 , CCL24/Eotaxin-2 , CCL26/Eotaxin-3 |
|
|
Cell Signaling Proteins |
Active Caspase-3 , AKT2 , AKT3 , AKT1 , ATF2 |
|
Cortisol, Apo A1 , BDNF , Cathepsin D |
|
|
Bone Metabolism Markers |
RANKL , Adiponectin , IL-1β , ACTH , Insulin |
|
Skin Growth Regulators |
Cortisol, Fibronectin , Hyaluronic Acid Synthase (HAS) |
|
Apoptosis & Sepsis-Related Factors |
|
|
CA-125 , IGF-II , Leptin , MIF , Osteopontin |
|
|
Endocrine Hormones |
|
|
Cardiovascular Factors |
Adiponectin , Apo A1 , IFN-γ , TNFα |
|
Renal Toxicity Markers |
Albumin , Clusterin , Cystatin C , KIM-1 |
|
Metabolic Factors |
Service Workflow

Service Details
-
Key Applications:
- Host cell proteins from expression systems
- Cell culture media protein analysis
- Pathogen-infected cell culture studies
- Detection of protein degradation products (cleaved, truncated, oxidized, or deamidated forms)
-
Advantages of iTRAQ Multiplex Analysis:
- Thousands of peptides identified and quantified in a single analysis
- Large dynamic range, capturing both high and low abundance proteins
- Broad protein diversity detected, including hydrophobic membrane proteins and high molecular weight proteins
Protein Quantification as a Foundation for Advanced Protein Analysis
Accurate protein quantification is not only essential for routine research and quality control, but also serves as the foundation for more advanced proteomic technologies. One example is the protein microarray, a high-throughput platform used to study protein–protein interactions, antibody profiling, and biomarker discovery.
Because protein microarrays rely on consistent and well-characterized protein inputs, reliable quantitation is a critical first step. By ensuring precise measurement of protein concentration, Creative BioMart helps set the stage for downstream applications such as:
- Functional protein screening
- Comparative expression analysis
- Diagnostic and biomarker research
If you are interested in expanding beyond protein concentration measurements, explore our Protein Microarray Services to see how quantitation supports deeper insights into protein function and interactions.
Advantages of Our Protein Quantitation Expertise
- Extensive Expertise: Years of proven success in protein research and assay development.
- Comprehensive Service Portfolio: From routine spectrophotometry to advanced LC-MS/MS.
- Regulatory Compliance: Services available under R&D, GLP, or GMP standards.
- Advanced Technologies: Use of state-of-the-art instruments including Biacore, Orbitrap, and QTOF systems.
- Customizable Solutions: Flexible packages for individual assays or complete analysis programs.
- Reliable Documentation: Strict data management and reporting systems for regulatory submissions.
Real-World Applications of Protein Quantitation
Case 1: Quantification of cytokine storms during virus infections
Yuan et al., 2021. Doi:10.3389/fimmu.2021.659419
Highly pathogenic viral infections often trigger cytokine storms, causing severe organ damage and high mortality. Interleukin-4 (IL-4) and interferon-γ (IFN-γ) are central regulators of these storms, yet their relative importance varies across viruses. To clarify this, data on cytokine fold change (FC), viral load, and clearance rates from infections such as SARS-CoV, MERS-CoV, SARS-CoV-2, influenza strains, Ebola, HIV, dengue, Zika, West Nile, hepatitis B and C, and enterovirus 71 were analyzed. Results revealed that IP-10, IL-6, IL-8, and IL-17 showed the largest induction amplitudes. Mathematical equations linking cytokine responses with viral dynamics were derived, highlighting potential clinical implications for cytokine-targeted therapies.
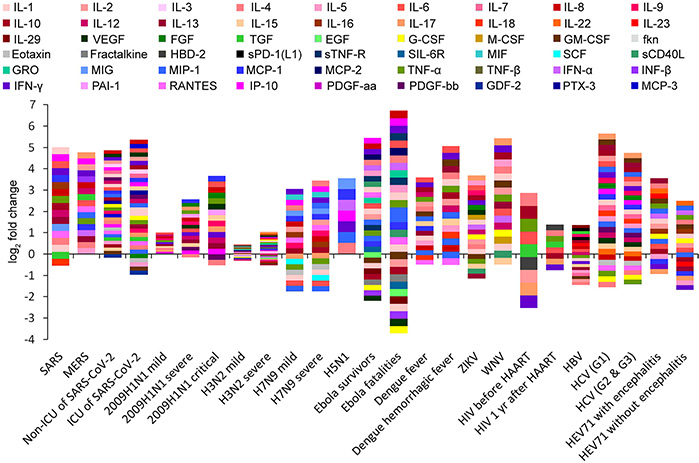
Figure 1 Cytokine profiling barcodes during different virus infections. ICU, intensive care unit; HAART, highly active antiretroviral therapy. (Yuan et al., 2021)
Case 2: Absolute protein quantitation of the mouse macrophage Toll-like receptor and chemotaxis pathways
Manes et al., 2022. doi:10.1038/s41597-022-01612-y
The Toll-like receptor (TLR) and chemotaxis pathways are essential for innate immunity, with ligand concentration, timing, and structure influencing downstream responses. To enable accurate computational modeling, researchers developed targeted mass spectrometry assays to measure absolute protein abundance in mouse macrophage TLR4 and chemotaxis pathways. Quantification, based on two peptides per protein when possible, revealed protein levels ranging from 1,332 to 227 million copies per cell. These values showed moderate correlation with transcript abundances from existing RNA-seq data, and integration of both datasets enabled proteome-wide abundance estimates. The resulting resource supports pathway modeling, simulation, and broader studies of immune regulation.
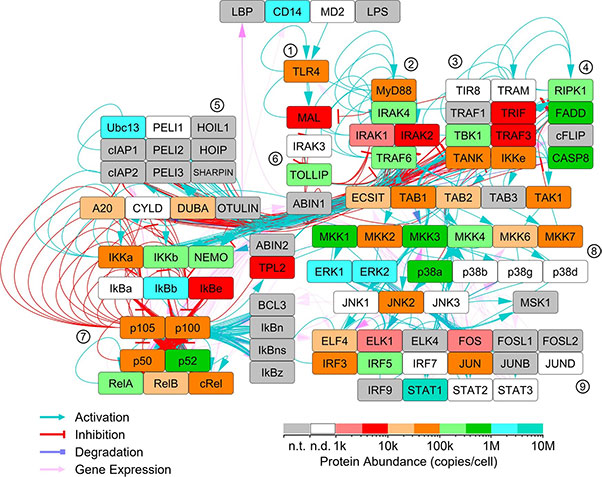
Figure 2. Quantitation of core TLR4 pathway proteins within mouse BMDMs. LC-PRM assays were performed to quantitate proteins of the core TLR4 pathway. Some proteins were targeted but were not detected (white nodes), possibly because their abundance was below the limit of detection of the LC-MS. Selected clusters of nodes are indicated by encircled numbers: 1. TLR4 receptors, 2. Myddosome signaling complex, 3. Triffosome signaling complex, 4. programmed cell death, 5. K63 and M1 polyubiquitination, 6. negative regulators, 7. NFκB pathway, 8. MAP kinase signaling, 9. transcription factors. (Manes et al., 2022)
Researcher Experiences with Our Quantitation Services
"We engaged Creative BioMart to support our Phase II clinical trial by quantifying cytokine biomarkers in patient serum samples. Their ability to detect low-abundance targets such as IL-17 and IP-10 with precision was critical to validating our therapeutic’s mechanism of action. The team’s strict adherence to GLP standards ensured our data met regulatory expectations, and the final report integrated seamlessly into our submission package. Their expertise gave us confidence at a pivotal stage of our program."
— Clinical Development Director | Mid-Sized Biotech Company
"In our preclinical safety assessment, Creative BioMart provided protein quantitation services for renal toxicity markers including albumin, clusterin, and KIM-1. Their thorough workflow allowed us to track subtle protein changes over time, which directly informed our dosing strategy. The combination of mass spectrometry and spectrophotometric assays provided both sensitivity and robustness. Their rapid turnaround and detailed documentation made a significant difference in keeping our IND timeline on track."
— Head of Toxicology | Contract Research Organization (CRO)
"Our discovery team needed high-throughput protein profiling to explore cytokine and chemokine responses in multiple cell-based assays. Creative BioMart’s iTRAQ multiplex quantitation service identified thousands of peptides in a single run, including several low-abundance signaling proteins we had struggled to detect with in-house methods. Their bioinformatics support was excellent—helping us interpret complex expression patterns across treatment groups. This partnership provided new insights that shaped the direction of our lead candidate program."
— Director of Discovery Biology | Global Pharmaceutical Company
"We have worked with Creative BioMart on multiple protein quantitation projects over the past three years, ranging from routine BCA protein assays to advanced LC-MS/MS-based multiplex analysis. In one project, their team successfully validated an assay for tumor biomarkers such as CA-125 and osteopontin under GMP conditions, which we now use as part of our oncology pipeline. Their flexibility, professionalism, and consistent quality have made them a trusted extension of our internal research team."
— Senior Scientist | Academic Medical Research Institute
FAQs About Protein Quantitation Service
-
Q: What types of proteins can you quantify?
A: We support a broad range of proteins, including cytokines, chemokines, growth factors, immunoglobulins, tumor biomarkers, endocrine hormones, cardiovascular markers, renal toxicity indicators, and metabolic factors. Whether you need routine concentration measurement or high-throughput multiplex profiling, our service portfolio ensures comprehensive coverage. -
Q: Which quantitation methods do you use?
A: We offer multiple platforms tailored to project needs:- Spectrophotometric methods (Bradford, BCA, Biuret, Lowry, OD600, absorbance, transmittance) for standard protein concentration measurement.
- Functional activity assays (enzyme activity, transcription factor assays, promoter methylation arrays, etc.).
- Advanced LC-MS/MS approaches such as iTRAQ multiplex quantitation, capable of identifying thousands of peptides in one analysis with high sensitivity and a wide dynamic range.
-
Q: Can your assays be performed under regulatory compliance?
A: Yes. Assays can be developed, verified, qualified, or fully validated under R&D, GLP, or GMP conditions, depending on your project needs. Our strict documentation and quality management systems ensure all data are regulatory-ready. -
Q: How do you ensure accuracy and reproducibility?
A: We use state-of-the-art platforms including Biacore (SPR), Orbitrap, and QTOF mass spectrometers, combined with optimized protocols and bioinformatics pipelines. Our dual-layered quality control—instrument performance checks and cross-platform validation—guarantees both sensitivity and reproducibility. -
Q: What is the typical turnaround time?
A: Turnaround time depends on assay complexity. Standard protein quantitation assays are typically completed within 1–2 weeks, while multiplex LC-MS/MS projects may take 3–5 weeks. We always provide a detailed timeline during project consultation. -
Q: Do you offer customizable service packages?
A: Absolutely. Clients can choose individual assays (e.g., cytokine quantitation) or comprehensive protein analysis packages to fulfill regulatory and research requirements. We tailor every project to fit specific objectives, budget, and regulatory expectations.
Resources
Related Services
- Host Cell Protein Mitigation
- Protein Expression Microarray
- Protein Expression and Purification Services
- Protein Characterization
- Membrane Protein Screening
- GLP-Compliant Bioprocess Services
- Protein Microarray Service
Related Products
References:
- Manes NP, Calzola JM, Kaplan PR, et al. Absolute protein quantitation of the mouse macrophage Toll-like receptor and chemotaxis pathways. Sci Data. 2022;9(1):491. doi:10.1038/s41597-022-01612-y
- Yuan S, Jiang SC, Zhang ZW, Fu YF, Hu J, Li ZL. Quantification of cytokine storms during virus infections. Front Immunol. 2021;12:659419. doi:10.3389/fimmu.2021.659419
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

