Protocol of Stable Cell Line Generation
Background
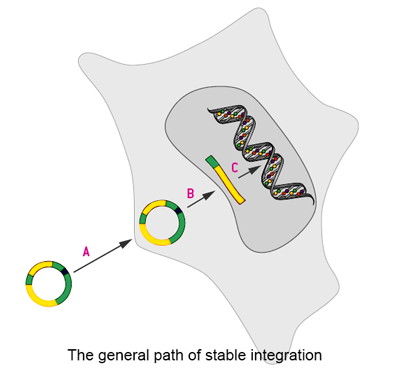
The generation of stably-transfected cell lines is critical for a wide range of applications. It is applied to the production of recombinant proteins, gene function studies, as well as drug discovery assays. Stable expression of target gene in cell lines overcomes the low transfection efficiency of transient expression and produces more target proteins.
There are two types of stable cell lines. One is achieved by eukaryotic vectors that harbor elements for episomal maintenance in the nucleus of a transfected cell. Another is achieved via direct integration of the transfected plasmid into the target cells genome. Episomal stability is often limited and episomal plasmid elements is often restricted to certain species, therefore integration into the host cell chromosome is in common use. Although integration into the host cell chromosome is a rare event and, for most purposes, clonal events have to be isolated, stability of the intended genetic modification usually is much higher.
Major challenges for generation of stable cell lines are low transfection efficiency and/or integration frequency. Stable expression can be influenced by the transfection method used. The transfection method determines the cell type for stable integration. It is known that liposome reagents can be used to transfer DNA into adherent cell lines. While viral methods or electrotransfection are used to deliver DNA into primary cells or notoriously difficult-to-transfect suspension cell lines. Unfortunately, viral methods suffer from several limitations, such as time consuming production of vectors and safety concerns, while electrotransfection suffer from the low cell survival rate.
Selection Marker
In order to select stably-transfected cells, a selection marker must be co-expressed with the target protein. The marker gene could be on either the same plasmid vector or a second, co-transfected vector. There are a variety of systems for selecting transfected cells, including resistance to antibiotics puromycin, neomycin, DHFR, and glutamine synthetase. After gene transfer, cells are developed in medium containing the selective agent. Only those cells which have contained the drug resistant gene survive.
Methods to Generate Stable Cell Lines
Depending on the scope of the experiment, several options are used for the generation of a stable cell line. A mixed population of drug resistant cells can be used directly for experimental analysis with the advantage of generating fast results, but also the disadvantage of dealing with an undefined and genetically mixed cell population. Another option is to generate a monoclonal cell line. In this method, it is necessary to dilute the resistant cells by plating in 96-well plates in such a way that culture as single and isolated cells. Subsequently, the cloning of single cell may be repeated several times to obtain 100% clonal purity. This culture method can be used for screening experiments or conduction studies by using a homogenous and defined cell system.
In conclusion, depending on the type of expression you’re interested in and the construct that you are incorporating, there are many different approaches for generation of stable cell lines. This protocol is specific for the generation of a monoclonal cell line that resistance to antibiotics G418 (neomycin). The end result that you are looking for is a population of cells in which 100% of cells are expressing your fusion protein.
Culture Conditions
Culture conditions (passage, split rhythm, number, etc.) of your selected cell type are critical for generation of stable cell lines. For optimal results, we recommend following the cell culture recommendations of the supplier (e.g. ATCC) for the respective cell type. In general, for promoting good proliferation and cell physiology, the cell line should be passaged two days before the experiment. Besides, cell passage should not be higher than 30 due to the possibility of interference with integration efficiency.
Experimental Outline
Design experiment
Design experiment and choose expression vector, cell type and transfection method. Make sure that your expression vector and transfection method are suitable for your cell type.
Choose the G418 concentration and cell number
Determine appropriate G418 concentration and cell number per well by matrix titration. Pay attention to the active concentration of stock G418 is vary from batch to batch.
Transfection
Transfect expression plasmid into cells. Pease follow the manufacturer’s instruction of your transfection system. Do not add G418 to culture medium immediately after transfection.
Cell selection
Plate transfected cells and cultivate cells in medium with G418 in appropriate concentration. A mixed population of drug resistant cells is obtained.
Monoclonal cell line screening
Dilute cells into 96 well culture plates in appropriate cell density per well. Feed every 10 days with selection medium. Refreshed selection medium is important to avoid false positive cells.
Analyze
Make sure to choose a suitable method for your application to analysis your stable-transfected cells.
Protocol
1. Choose the G418 Concentration
Susceptibility to G418 is different among cell lines, which many even vary with cell passage numbers. The selection condition (e.g. G418 concentration, plating density) for your specific cell type needs to be determined experimentally. Determine the minimum level G418 concentration to guarantee the minimum impact to cell growth. Note that the active concentration of stock G418 can vary considerably from batch to batch. Therefore, we recommend testing G418 sensitivity for every new batch. The final plating density depends on the specific cell type and the G418 concentration. We therefore recommend matrix titration of G418 and titration of cell number for determination of plating density in one 96-well plate.
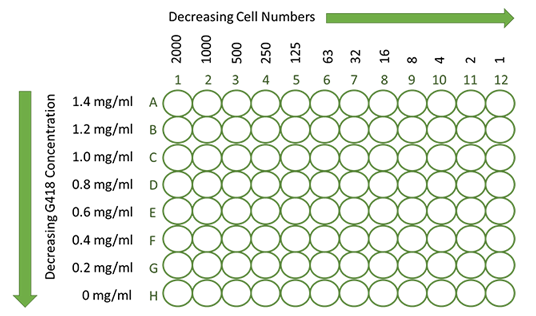
- Pre-plate 100 μl medium in each well of the plate.
- Add 100 μl of cell suspension containing 4000 cells per well to the first column (#1).
- After gentle up and down pipetting, carry over 100 μl to the next column, thereby diluting in a ratio of 1:2. Repeat this procedure for each consecutive column.
- Discard 100 μl from the last column (#12) after completing. The first column should then contain about 2000 cells per well, the last column contain around one cell per well on average.
- Add 100 μl of G418 containing medium (2.8 mg/ml) to the first row (A) for a final G418 concentration of 1.4 mg/ml.
- Add G418 to the following rows in decreasing concentrations of G418 in steps to 0.2 mg/ml. For the last row (H) add medium without G418.
- Incubate cells at standard conditions.
- Analyze cell growth by microscope. In some cases, cell growth can also be observed by change of medium color.
- If you observe cell growth (after 10 days) in the wells without G418, it is reasonable to assume that those cells can grow out starting as single cells.
- Choose the G418 concentration which is just above the one which shows complete cell death as the appropriate G418 concentration for selection.
2. Transfection
For transfection, please follow the manufacturer’s instruction of your transfection system. The important thing is to transfect the expression plasmid containing the target gene and the sequence for a drug resistance gene into your cells. We suggest setting a negative control of untransfected cells for selection. Besides, it is much better to check the transfection efficiency and integration frequency of your experiment with a GFP-control plasmid.
3. Cell Selection Post-Transfection
- After transfection, allow cells to grow and to express the protein for G418 resistance under non-selective conditions for about 24-48 hours (reach the peak of protein expression according to your transfection system).
- Use suspension cells directly or trypsinize adherent cells for analysis. Analyze for transfection efficiency 24-48 hours post-transfection by microscopy or western blot of your target protein and positive control.
- Count living cells via trypan blue staining or other appropriate methods.
- Using standard medium and the appropriate amount of G418 pretested for your cell type, plate cells in a 96-well plate with different cell numbers per well (e.g., 0.5, 1, 2, 5, 10) in a volume of at least 100 μl per well. Depending on cell concentration determined before, conduct several serial dilution steps as applicable. It is important to thoroughly suspend cells before seeding, but avoid harsh treatment by frequent pipetting.
- Incubate cells under selective conditions and feed cells regularly with fresh selection medium.
- Cell clones can be analyzed or further expanded as soon as cells in the non-transfected control wells have completely died.
- In order to help assuring that selected cell populations are clones derived from a single cell, another round of limiting dilution under selection is recommended.
4. Analysis of Stable Cell Lines
Once you have obtained resistant cell lines, you should expand the cells and assay your target gene compared with positive and negative control. You can detect the expression of fusion protein by an appropriate analysis method such as western blot, microscopy, ELISA, as well as flow cytometry.
Creative BioMart provides stable cell line services to meet your specific needs. Not only have we developed cell lines stable at expression level, but also established stable cell lines ready for assay development.

