Luciferase Reporter Assay
Background
Luciferase is a class of oxidative enzymes that catalyze bioluminescent reactions, distinct from photoproteins. It is widely used as a sensitive reporter system due to its high sensitivity and minimal background interference. Compared to traditional assays like chloramphenicol acetyltransferase (CAT), luciferase offers a remarkable 1000-fold increase in sensitivity.
Figure 1. Structure of luciferase.
The Luciferase Reaction
The bioluminescent reaction catalyzed by firefly luciferase proceeds in two key steps: adenylation and oxidation. First, luciferin is activated by ATP, forming luciferyl adenylate. In the second step, this activated intermediate reacts with molecular oxygen, producing oxyluciferin and emitting visible light as a byproduct of the highly efficient oxidative process.
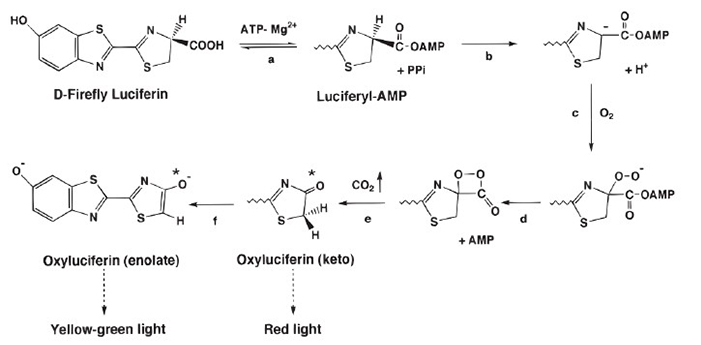
Figure 2. The firefly luciferase bioluminescent reaction. (Ripp et al., 2011)
Luciferase Production and Detection
Luciferase can be produced in the lab through genetic engineering. Luciferase genes can be synthesized and inserted into organisms or transfected into cells. Various organisms, including mice, silkworms, and potatoes, have been engineered to produce luciferase.
The emitted light from the luciferase reaction can be detected using sensitive apparatus such as luminometers or modified optical microscopes. This allows for the observation of biological processes in real-time.
At Creative BioMart, our Luciferase Reporter Assay Service enables real-time, quantitative monitoring of gene regulation events in a wide variety of biological samples. Using state-of-the-art luminometry and robust assay protocols, we help researchers accurately track luciferase expression from transfected plasmids, stable cell lines, or engineered organisms.
Whether you’re performing transcriptional activity studies, drug screening, or signal pathway mapping, we provide the technical excellence and customization needed for publication-quality results.
What We Offer?
Service Procedure
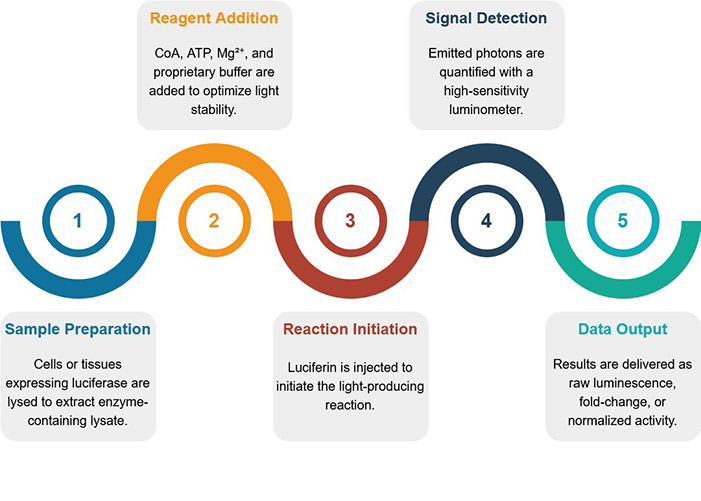
Advantages of Our Services
- High Sensitivity: Luciferase assays can detect as little as 0.02 pg of substrate, providing highly sensitive and accurate measurements.
- Minimal Background: Since no light excitation is required, autofluorescence is minimal, resulting in virtually background-free fluorescence.
- Versatile Applications: Luciferase can be engineered for various purposes, including gene regulation studies, protein-protein interactions, and drug screening.
Why Choose Us?
- Ultra-Sensitive Detection: Detect minute amounts of reporter activity with near-zero background interference.
- Reliable, Quantitative Output: Linear luminescence response across a broad dynamic range.
- Cost-Effective & Fast Turnaround: Get high-quality data without breaking the budget—results delivered on time.
- Expert Support: Our scientists provide consultation to help interpret results and design follow-up experiments.
- Customizable Formats: Flexible assay setups tailored to your vector system, promoter, or experimental goal.
- High Compatibility: Seamless integration with various cell lines, reporter constructs, and detection platforms.
Case Study
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: A rapid luciferase reporter assay using a RUNX2 promoter
Wang et al., 2021. doi:10.3389/fcell.2020.607383
This study addresses the need for faster, cost-effective methods to screen plant-based compounds for osteoporosis (OP) treatment. Researchers developed a rapid luciferase reporter assay using a RUNX2 promoter—a key transcription factor in osteogenesis—integrated into mouse mesenchymal stem cells (C3H10T1/2). Eight natural compounds were screened for osteogenic activity and compared to the known phytoestrogen daidzein. Results from this platform showed strong correlation with traditional in vitro and in vivo osteogenesis assays using mouse and human bone marrow MSCs. The new method reduced screening time from weeks to days, making it a reliable, high-throughput tool for identifying potential OP therapeutics.
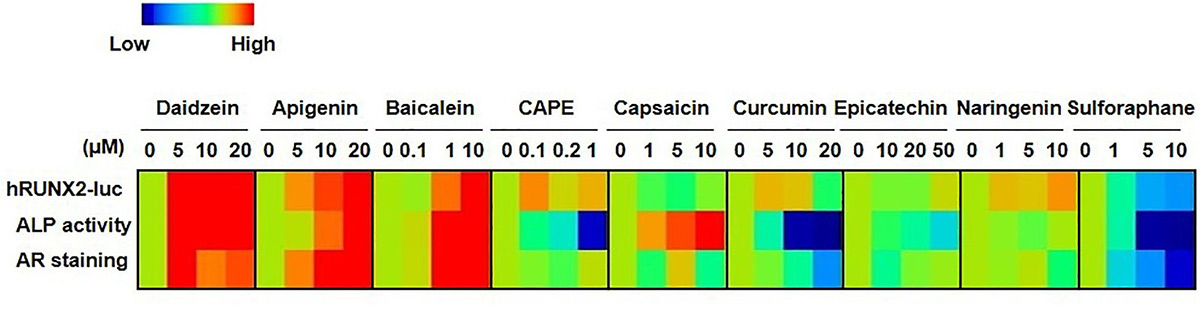
Figure 3. Comparisons of hRUNX2-luc reporter activity in C3H MSCs to in vitro functional assays demonstrate that hRUNX2-luc reporter activity but not ALP activity is highly correlated with in vitro mineralization assay. Heatmap analysis of expression levels of hRUNX2-luc reporter activity in compound-treated C3H MSCs, and ALP activity as well as AR staining in primary murine BMMSCs treated with tested compounds. (Wang et al., 2021)
Case 2: Serum amyloid A3 promoter-luciferase reporter mice are useful for early drug-induced nephrotoxicity detection
Kudo et al., 2024. doi:10.3390/ijms25105124
This study highlights a novel, non-invasive approach for early detection of drug-induced kidney injury using Saa3 promoter-luciferase reporter mice. After repeated low-dose injections of aristolochic acid (AA) and cisplatin (Cis)—both nephrotoxic agents—researchers observed elevated renal mRNA markers of inflammation and injury. In vivo bioluminescence imaging showed that injured kidneys emitted strong signals, specifically linked to Saa3 promoter activation. Opening the abdominal cavity confirmed that the bioluminescent signal originated directly from the damaged kidneys. These findings suggest that Saa3 promoter activity is a reliable, non-invasive biomarker for early-stage nephrotoxicity, valuable in drug development and toxicity screening.
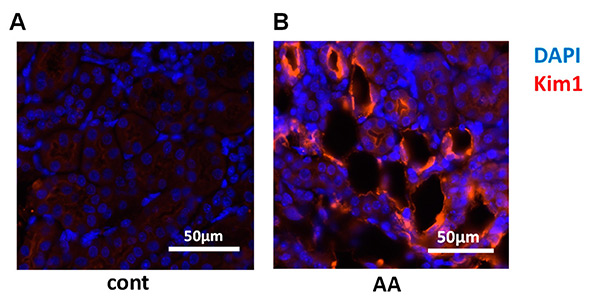
Figure 4. Visualization of renal pathology in multiple low-dose aristolochic acid (AA) model using Saa3 promoter-luc mice. (A,B) Representative images of injured renal tubules from kidney stained for Kim1 (red) and DAPI (blue) immunoreactivity. (Kudo et al., 2024)
Customer Testimonials
-
"We used Creative BioMart’s luciferase reporter assay service to assess pathway activation in our oncology target validation program. The results were highly reproducible, with excellent signal-to-noise ratio, even in low-expressing cell lines. The team provided clear data reports and was quick to troubleshoot assay optimization. Their scientific insight and responsiveness really stood out."
– R&D Director | Global Pharmaceutical Company
-
"These assays offered by Creative BioMart helped us confirm NF-κB activity in response to inflammatory stimuli. The turnaround time was fast, and the technical team worked with us to customize the reporter constructs. We appreciated their flexibility in working with primary cell models, which can be technically challenging."
– Principal Investigator | Academic Medical Center
-
"We screened over 300 small molecules using a luciferase reporter assay to detect CREB pathway activation. The assay was scalable, and the results were delivered in a format ready for immediate analysis. Thanks to Creative BioMart’s QC metrics, we had confidence in the hits we identified for follow-up."
– Senior Scientist | Biotech Startup Focused on CNS Disorders
-
"We required robust transcriptional activity data for a pre-IND package, and Creative BioMart’s luciferase reporter assay service delivered exactly what we needed. The results were well-documented, reproducible across replicates, and accepted without question by our regulatory reviewers. The service saved us both time and internal resources."
– Project Manager | Contract Research Organization (CRO)
-
"We needed a custom dual-reporter assay to monitor promoter activity under hypoxic conditions. The Creative BioMart’s team cloned and validated our construct quickly and provided detailed validation data. The resulting assay enabled us to track subtle transcriptional changes with confidence. Highly recommend for any custom reporter needs."
– Molecular Biology Team Lead | Government Research Institute
FAQs
-
Q: What types of reporter systems do you offer?
A: We offer a range of luciferase-based assays, including firefly, Renilla, and dual-luciferase systems, depending on your experimental needs. Dual-reporter assays are ideal for normalizing transfection efficiency or internal controls, and we can also design custom reporter constructs to target specific pathways or promoters. -
Q: Can you handle high-throughput screening projects?
A: Yes! Our platform supports 96- and 384-well formats, making it fully scalable for high-throughput drug screening, transcriptional profiling, or compound efficacy testing. We provide fast turnaround and high reproducibility, even across large sample sets. -
Q: What kind of data quality can I expect?
A: Our assays are optimized for high signal-to-noise ratios, excellent sensitivity, and minimal background. We perform rigorous quality control at every step, and our customers consistently report strong reproducibility across replicates and batches. Data is delivered in a publication- and submission-ready format. -
Q: Do you offer custom assay development?
A: Absolutely. We specialize in custom reporter design, including cloning your promoter or response element of interest into luciferase vectors. Whether you're studying transcription factors, signaling pathways, or compound effects, we can tailor the assay to your exact specifications. -
Q: Can you work with difficult or primary cell lines?
A: Yes. Our team has extensive experience optimizing luciferase reporter assays for primary cells, stem cells, and low-transfection-efficiency cell lines. We’ll work closely with you to adjust transfection conditions, vector design, and detection sensitivity as needed. -
Q: How do you ensure assay reproducibility?
A: We use standardized protocols, triplicate runs, and internal controls to ensure consistency. Our dual-luciferase setups allow for normalization, and we include raw data, QC metrics, and interpretation notes in every report.
Resources
Related Services
- Transcription Factor Assay
- Enzyme Activity Assay
- Drug Discovery Screening
- Transporter Screening Assays
- GPCR Screening Assays
- Ion Channel Screening Assays
- Protein Pathway Profiling
- Epitope Mapping
Related Products
References:
- Kudo A, Osedo H, Aisyah R, et al. Serum amyloid A3 promoter-luciferase reporter mice are useful for early drug-induced nephrotoxicity detection. IJMS. 2024;25(10):5124. doi:10.3390/ijms25105124
- Ripp S, Sayler G, Close D. Mammalian-based bioreporter targets: protein expression for bioluminescent and fluorescent detection in the mammalian cellular background. In: Serra PA, ed. Biosensors for Health, Environment and Biosecurity. InTech; 2011. doi:10.5772/17028
- Wang LT, Lee YW, Bai CH, et al. A rapid and highly predictive in vitro screening platform for osteogenic natural compounds using human RUNX2 transcriptional activity in mesenchymal stem cells. Front Cell Dev Biol. 2021;8:607383. doi:10.3389/fcell.2020.607383
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

