Protocol of the Phosphorylated Antibody Detection Method
Protein phosphorylation is a very important and common phenomenon in eukaryotes. Among the three most common phosphorylation forms in eukaryotes, serine phosphorylation is the most, threonine phosphorylation is the second, and tyrosine phosphorylation is the least. The ratio of the three is 800:200:1. Western blot with anti-phosphate amino acid antibody and phosphorylated protein is one of the commonly used methods to detect phosphorylated protein. There are now commercial antibodies against tyrosine, serine and threonine phosphate, of which the anti-tyrosine phosphate antibody is the most specific and commonly used. The antigenic determinant of phosphorylated serine and threonine is small, and the antigen-antibody binding site has space barrier, so the binding force of antibody is poor. In this section, the anti-tyrosine phosphate antibody is used as the detection antibody, and the protein on the SDS-PAGE gel of two-dimensional electrophoresis is used as the protein sample for detection.
This experimental manual is designed to help scientific researchers master the basic principles and main steps of western blot detection of protein phosphorylation level, and understand the application of phosphorylation antibody detection methods.
After the protein is phosphorylated, the phosphoamino acid can be identified by the method of biosynthetic antibody. For example, the rare phosphotyrosine contained in the protein can be detected by anti-phosphotyrosine antibody, with high specificity and sensitivity. The phosphorylated tyrosine amino acid residues were detected by direct method, that is, after the imprinted film was transferred and sealed, the antibody against phosphorylated tyrosine amino acid residues was used for detection, and the signal was detected by western blot standard detection method. The specificity of this method is very good, and it can confirm whether the detected bands are phosphorylated.
After the completion of two-dimensional electrophoresis, the membrane transfer detection is carried out. The isoelectric focusing electrophoresis is carried out in the first direction of two-dimensional electrophoresis, and then the SDS-PAGE is carried out in the vertical direction; Separate proteins according to their relative molecular weight, so that different proteins can be separated as much as possible.
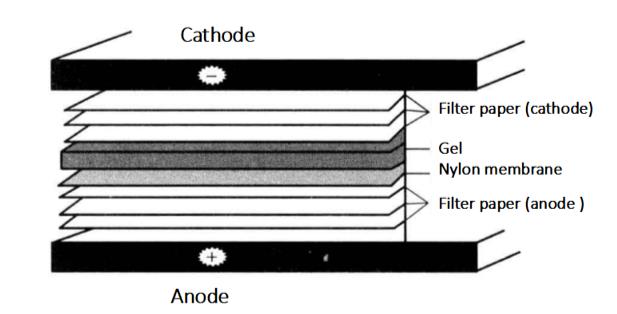
The transfer membranes commonly used in western blot are mainly nitrocellulose blocking membranes (NC) and PVDF membranes (polyvinylidene-fluride). In addition, DEAE cellulose membranes and nylon membranes can also be used. The selection principles are as follows.
(1) The abipty of the membrane to bind to the target protein molecule (that is, the amount of protein that the membrane can bind to per unit area).
(2) The pore size of the membrane (that is, the size of the intercepting protein).
(3) It does not affect the subsequent signal detection (good signal-to-noise ratio).
(4) If there are other requirements for subsequent experiments, such as protein sequencing or mass spectrometry, different transfer membranes should be selected according to different purposes.
(5) The types and apppcation scope of membrane are shown in the table below.
| NC membrane | Nylon membrane | PVDF membrane | |
| Sensitivity and Resolution | High | High | High |
| Background | Low | High | Low |
| Binding capacity (μg/ cm2) | 80-110 | >400 | 125 -200 (suitable for binding to proteins in the presence of SDS) |
| Material texture | Dry NC film is brittle | Soft and firm | High mechanical strength |
| Solvent tolerance | None | None | Yes |
| Operating procedure | Wetting with buffer to avoid air bubbles | Wetting with buffer | 100% methanol wetting before use |
| Scope of application | 0.45μm: general proteins 0.2μm: proteins with relative molecular mass less than 2000 0.1μm: proteins with relative molecular mass less than 7000 |
Low concentration of small molecule proteins, acidic proteins, glycoproteins and proteoglycans. | Glycoprotein detection and protein sequencing. |
| Detection method | Routine staining with radioactive and non-radioactive detection. | Anionic dyes cannot be used. | Routine staining, comparable to NC membranes, available in Coomassie Brilliant Blue for ECL assays and rapid immunoassays. |
| Price | Cheap | Cheap |
High |
1. Main Instruments and Equipment
Micropipette, micro centrifuge, 1.5mL centrifuge tube, 50mL centrifuge tube, thermostatic water bath, ice maker, shaker, oscillator, centrifuge, metal bath, polyacrylamide gel electrophoresis device, autoradiography box, X-ray film, hybrid bag, PVDF membrane, filter paper.
2. Material
Protein samples extracted by various methods, tyrosine phosphorylation antibody, Whatman 3 mm filter paper.
3. Main reagents
(1) 6×SDS loading buffer
| 300 mmol/L | Tris-HCl (pH 6.8) |
| 600 mmol/L | DTT |
| 12% | SDS |
| 60% (Volume fraction) | Glycerol |
| 0.6% | Bromophenol blue |
(2) Cathodic buffer (pH9.4)
| 25 mmol/L | Tris-HCl |
| 40mmol/L | Glycine |
| 10% (Volume fraction) | Methanol |
(3) Anode buffer I (pH10.4)
| 0.3mmol/L | Tris-HCl |
| 10% (Volume fraction) | Methanol |
(4) Anode buffer II (pH10.4)
| 25 mmol/L | Tris-HCl |
| 10% (Volume fraction) | Methanol |
(5) Tris-buffered saline, TBS
| 100mmol/L | Tris-HCl (pH 7.5) |
| 0.9% (Volume fraction) | NaCl |
(6) LumiGLO mixture
| 0.5mL20× | LumiGLO |
| 0.5mL20× | H2O2 |
| 9.0mL | H2O |
(7) Hydrated solution
(8) Balance buffer
(9) Capped agarose solution
(10) Tris-glycine electrophoresis buffer X.
(11) 12% SDS-PAGE gel.
(12) TBST (Tris-buffered sapne tween-20) buffer: 0.1% (volume fraction) tween-20 is added to TBS.
(13) Blocking buffer: 5% skimmed milk powder is added to TBST.
(14) First anti-dilution buffer: TBST of 5% BSA.
(15) Second anti-dilution buffer: TBST of 5% skimmed milk powder.
(16) Stationary solution.
(17) Coomassie brilpant blue staining solution.
(18) Coomassie brilliant blue bleaching solution.
1. Protein separation
Total protein extracted by modified phenol method.
2. Protein lysis
Protein lysis by lysis solution.
3. Two-dimensional electrophoresis
In order to separate as many phosphorylated proteins as possible, we used 24 cm adhesive strips, pH 3-10, and made two parallel pieces of adhesive for each sample during electrophoresis, one for Kemas Brilliant Blue staining. One for semi-dry transfer and one for western blot detection.
4. western blot detection of protein phosphorylation levels
(1) Semi-dry transfer
① At the end of electrophoresis, cut off the gel*1 of appropriate size, one piece was used for Kemas Brilliant Blue staining and one piece was put into Towbin buffer and removed after 15 min for western blot.
② Cut six pieces of Whatman 3 mm filter paper of the exact same size as the gel and one PVDF mem/pane, and mark the corner of the mem/pane with a soft pencil.
③ Add 2 Whatman 3mm filter paper to anode buffer I; Add a Whatman 3 mm filter paper to the anode buffer II; Add three Whatman 3 mm filter paper to the cathode buffer.
④ The PVDF mem/pane is soaked in methanol until translucent, and then transferred to aqueous solution for 2min. Put it into anode buffer II for at least 5min.
⑤ Clean the cathode and anode plate with distilled water and keep them wet.
⑥ On the bottom graphite electrode (anode) of the electrorotator, cover two Whatman3mm filter papers soaked in anode buffer I in turn, then cover one Whatman3mm filter paper soaked in anode buffer II, PVDF film, gel and three Whatman3mm filter papers *2 soaked in cathode buffer.
⑦ Install the upper electrode (cathode) of the electrometer, connect the power supply, and rotate the film. Calculate the electric flow according to the membrane area mA=membrane area × (2~2.5) (If the relative molecular weight of protein is small × 2. If the relative molecular weight of protein is large × 2.5), turn on the membrane for 1h *3.
⑧ After the electric rotation is finished, remove the film and set aside*4.
(2) Blocking
① Prepare blocking buffer: 1 × TBS solution is TBST solution by adding 0.1% (volume fraction) tween-20.
② Take 10mL of sealing solution and add 5% skimmed milk powder to make sealing solution.
③ Put the transferred membrane into the sealing solution and incubate it in the shaking bed for 1h.
(3) Primary antibody binding
① Remove the membrane and wash 3 times with TBST for 5 min each time.
② Take 10mL of primary antibody dilution on ice and add appropriate amount of tyrosine phosphorylation specific antibody*5, incubate on ice for 1h and shake gently overnight.
(4) Secondary antibody binding
① The membrane was removed and washed 3 times with TBST for 5 min each.
② The secondary antibody for tyrosine phosphorylation specific antibody was adjusted to the appropriate concentration with secondary antibody dilution buffer, and the membrane was incubated for 1h at room temperature.
(5) Exposure imaging
① Wash the membrane 3 times with TBST for 5 min each time first.
② Prepare chromogenic solution LumiGlo Reagent A + 50μL Peroxide Reagent B and store it away from light until use.
③ Apply the appropriate amount of color developing solution to the membrane according to the size of the membrane, develop the color in the dark for 5 min, squeeze out the color developing solution, put it into the dark box with the membrane face up, place an X-ray film on the membrane in the dark room, and expose it for 2~30 min. After exposure, wash the film in the dark room and observe the results*6 (Figure 7-3-1).
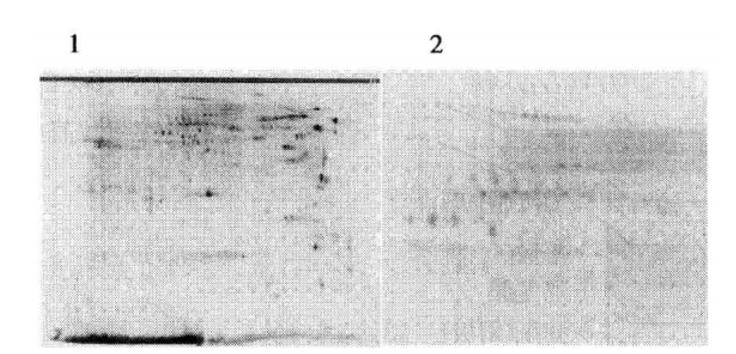
Figure 7-3-1 Western blot results with tyrosine phosphorylated protein as antibody (using mouse total protein as an example) 1: coomassie brilliant blue staining; 2: western blot assay results*7
1. Take care to adopt a suitable method to obtain a high pure amount of total protein, which is necessary to detect phosphorylated proteins by western blot.
2. When two-way electrophoresis is performed, care should be taken to prepare two samples in parallel and set up a control group.
3. When transferring proteins to membranes, care should be taken that the proteins are adequately transferred to the hybridization membrane.
4. When western blot signal detection, minimize the interference of background.
5. The selection of suitable adhesive strips and the prediction of the position of the target protein are very important for the success of the experiment.
*1 This test uses 24cm adhesive tape. The whole gel after two-way operation is very large, and it is difficult for the whole piece to rotate the membrane at the same time. It is suggested that the position of the target protein to be detected on the gel should be predicted before the test, so that it can be cut easily for detection.
*2 When touching the glue, membrane or filter paper with your hands, you should wear gloves, because the oil and secretion on the skin will affect the transfer of protein from the glue to the membrane. Air bubbles shall be eliminated between all membranes.
*3 Gently clean the bubbles with the test tube and cover the transfer tank.
*4 After the membrane has been transferred, the glue can be put into Coomassie brilliant blue staining solution for staining to detect whether the protein is completely transferred.
*5 Antibodies can bind to the membrane as proteins in electrotransfer. Therefore, the sensitivity of western blot depends on the blocking of potential binding sites of some unrelated proteins. Generally, it is better to use skimmed milk powder, but also serum albumin. If the non-specific background is still high, Tween-20 can be added to the blocking solution to make its final concentration 0.02%. Generally, the presence of Tween-20 does not affect the binding of antibody and target antigen.
*6 The content of phosphorylated protein accounts for a small proportion of the total protein content, so the sample loading amount should be as large as possible during two-dimensional electrophoresis, and the exposure time should also be appropriately extended.
*7 The results showed that phosphorylated protein signals distributed in strings were detected.

