Metabolism Assays
Background
Metabolism is the complex network of chemical reactions that sustain life, enabling organisms to convert nutrients into energy, build cellular structures, and eliminate waste. These processes are tightly regulated and interconnected, forming the foundation of physiological homeostasis. Disruptions in metabolic pathways are implicated in a wide range of conditions, including cancer, diabetes, obesity, and neurodegenerative disorders.
Metabolism assays are powerful tools used to monitor and quantify various aspects of cellular and organismal metabolism. They provide critical insights into energy production, biosynthesis, redox balance, and nutrient utilization. By measuring specific metabolic outputs—such as ATP levels, oxygen consumption, extracellular acidification, glucose uptake, or metabolite profiles—researchers can dissect the functional status of pathways like glycolysis, oxidative phosphorylation, and the TCA cycle.
At Creative BioMart, we specialize in cutting-edge Metabolism Assays tailored for applications in drug discovery, toxicology, cell biology, and systems biology. Whether you're identifying metabolic vulnerabilities in cancer cells or evaluating the impact of new drug candidates on cellular energy pathways, our assays provide the precision and depth you need to advance your research.
What We Offer?
1. In VitroAssays
-
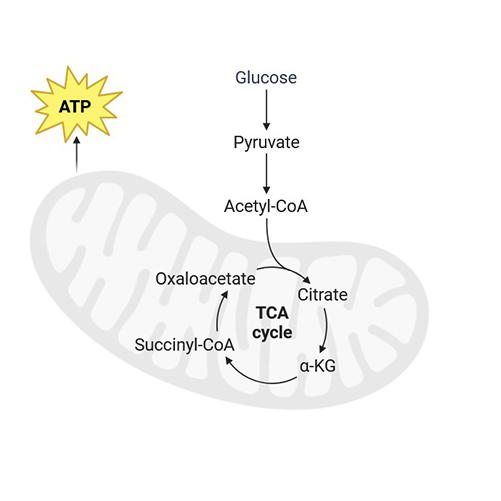
Cellular Respiration Assays
Measure oxygen consumption rates to assess mitochondrial function and cellular energy production.
-
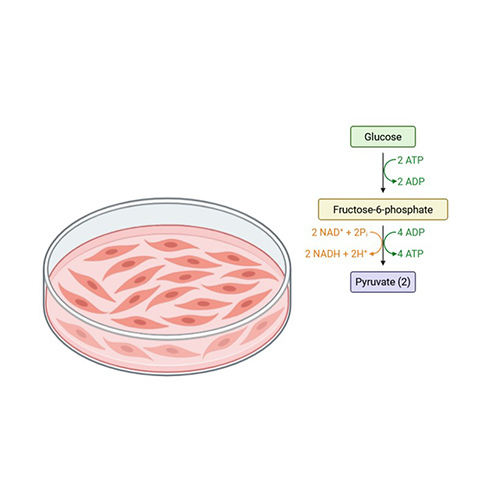
Glycolysis Stress Tests
Evaluate extracellular acidification rates to analyze glycolytic activity in live cells.
-
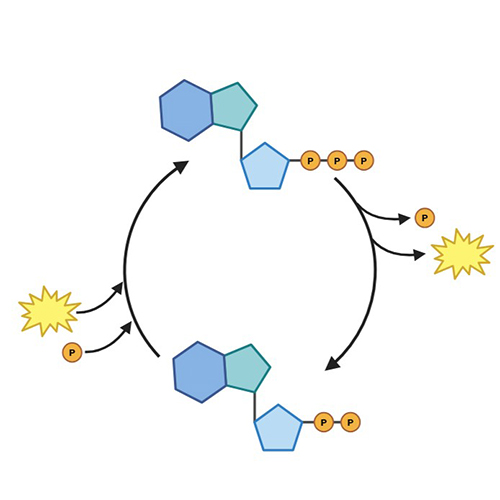
ATP Quantification Assays
Determine intracellular ATP levels to gauge cellular energy status.
-
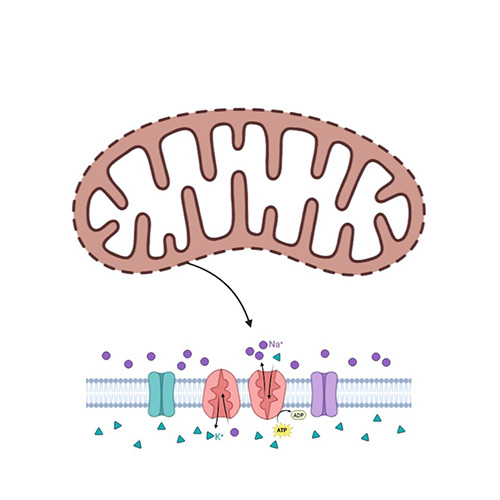
Mitochondrial Membrane Potential Assays
Assess the health of mitochondria by measuring membrane potential changes.
-

Reactive Oxygen Species (ROS) Detection
Quantify oxidative stress within cells by detecting ROS levels.
-
Interaction & Profiling
Assesses compound’s potential to inhibit CYP450 enzymes and determines specific CYP450 isoforms responsible for compound metabolism.
-
Metabolic Stability & Profiling
Identifies metabolic byproducts using LC-MS or other techniques to characterize compound breakdown.
-

Binding & Transport
Assesses how drugs interact with serum proteins, affecting bioavailability and distribution.
-
Metabolic Drug-Drug Interactions
Assesses metabolic pathway disruptions caused by co-administered compounds.
-
Species Comparison Capability
Tests drug metabolism in various species to support preclinical candidate selection.
-
Physicochemical Properties
Measures a compound’s solubility in aqueous solutions to predict absorption and formulation compatibility.
-
HERG Channel Inhibition
Evaluates the risk of cardiac arrhythmias by testing compound interference with HERG potassium channels.
2. Fatty Acids Related Assays
-
Fatty Acid Profiling
Identify and quantify various fatty acids in biological samples to study lipid metabolism.
-
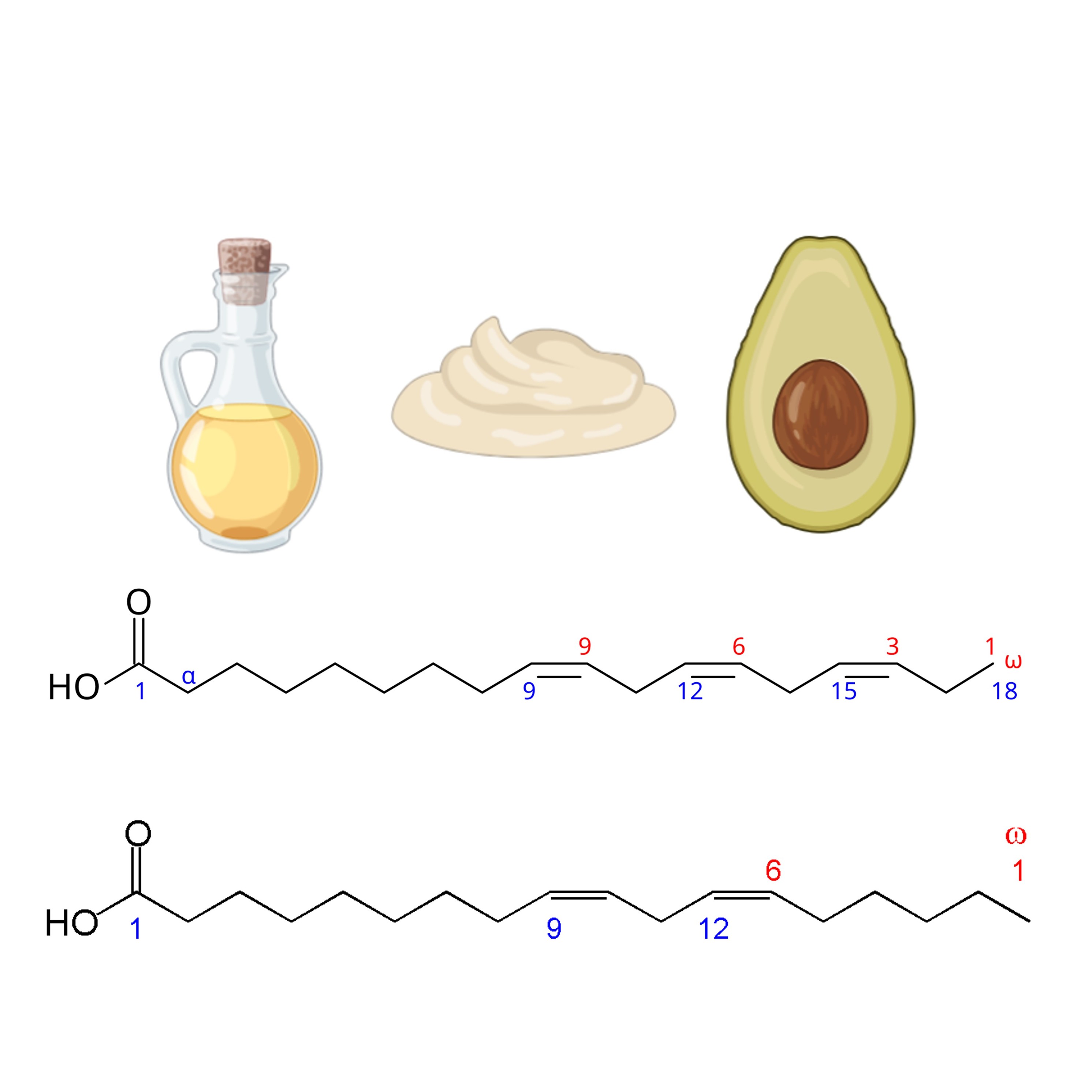
Omega-3 and Omega-6 Index Testing
Measure levels of essential fatty acids to assess nutritional status and inflammation risk.
-
Fatty Acid Oxidation Assays
Evaluate the rate at which cells oxidize fatty acids, indicating metabolic flexibility.
-
Fatty Acid Uptake Studies
Analyze the efficiency of fatty acid transport into cells.
-
Lipid Peroxidation Measurements
Detect oxidative degradation of lipids, an indicator of cellular damage.
3. Cholesterol/Cholesteryl Ester Quantification
-
Fatty Acid Profiling
Identify and quantify various fatty acids in biological samples to study lipid metabolism.
-

Omega-3 and Omega-6 Index Testing
Measure levels of essential fatty acids to assess nutritional status and inflammation risk.
-
Fatty Acid Oxidation Assays
Evaluate the rate at which cells oxidize fatty acids, indicating metabolic flexibility.
-
Fatty Acid Uptake Studies
Analyze the efficiency of fatty acid transport into cells.
-

Lipid Peroxidation Measurements
Detect oxidative degradation of lipids, an indicator of cellular damage.
Service Procedure

Why Choose Us?
- Comprehensive Assay Portfolio: From mitochondrial respiration to glycolysis, we cover the full spectrum of metabolism analysis to support diverse research goals.
- Flexible Project Design: We offer fully customizable workflows—from cell models and assay formats to data outputs—to fit your specific scientific and regulatory objectives.
- High-Throughput Capability: Accelerate your discovery timelines with scalable assay platforms designed for efficient screening of large compound libraries or conditions.
- Expert Scientific Support: Our experienced team partners with you at every step, offering guidance on assay selection, optimization, and data interpretation.
- Robust, Reproducible Data: We use validated protocols and rigorous QC standards to ensure consistent, high-quality results you can trust and publish with confidence.
- Rapid Turnaround Times: Stay ahead of deadlines with streamlined processes and responsive communication—because speed matters when science waits for no one.
Case Study
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: A rapid luciferase reporter assay using a RUNX2 promoter
Wang et al., 2021. doi:10.3389/fcell.2020.607383
This study addresses the need for faster, cost-effective methods to screen plant-based compounds for osteoporosis (OP) treatment. Researchers developed a rapid luciferase reporter assay using a RUNX2 promoter—a key transcription factor in osteogenesis—integrated into mouse mesenchymal stem cells (C3H10T1/2). Eight natural compounds were screened for osteogenic activity and compared to the known phytoestrogen daidzein. Results from this platform showed strong correlation with traditional in vitro and in vivo osteogenesis assays using mouse and human bone marrow MSCs. The new method reduced screening time from weeks to days, making it a reliable, high-throughput tool for identifying potential OP therapeutics.
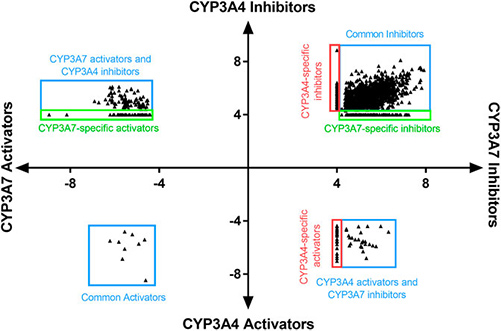
Figure 1. LogIC50 of CYP3A7 and CYP3A4 hits. (Kabir et al., 2022)
Case 2: Comprehensive metabolite profiling of Berdav propolis using LC-MS/MS
Karagecili et al., 2023. doi:10.3390/molecules28041739
This study analyzed the chemical composition and antioxidant potential of propolis, a plant-derived bee product rich in polyphenols and flavonoids. Using several antioxidant assays (ABTS•+, DPPH•, DMPD•+), propolis demonstrated strong radical scavenging and reducing capabilities, comparable to standard antioxidants like BHT, BHA, Trolox, and α-tocopherol. Propolis also showed notable enzyme inhibition against α-glycosidase, acetylcholinesterase (AChE), and carbonic anhydrase II (hCA II), with IC50 values suggesting potential therapeutic use for diabetes, Alzheimer’s disease, and glaucoma. LC-MS/MS identified 28 key phenolic compounds, including chrysin, quercetin, and caffeic acid. Overall, propolis extract shows promise as a natural antioxidant and multi-target bioactive agent.
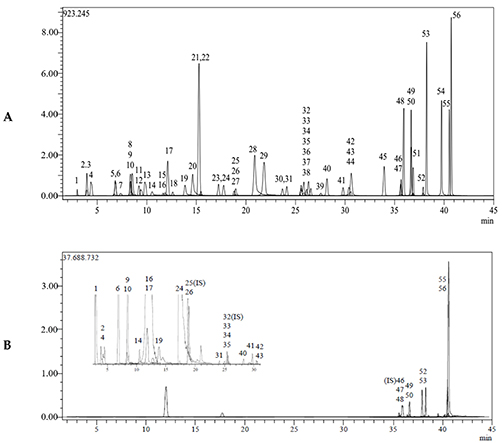
Figure 2. (A). Chromatogram of all standard phenolic compounds analyzed using the LC-MS/MS method 1: Quinic acid, 2: Fumaric acid, 4: Gallic acid 6: Protocatechuic acid, 9: Chlorogenic acid, 10: Protocatechuic aldehyde, 14: 4-OH Benzoic acid, 16: Vanillic acid, 17: Caffeic acid, 19: Vanillin, 24: p-Coumaric acid, 26: Ferulic acid, 31: Miquelianin, 33: Rutin, 34: isoquercitrin, 35: Hesperidin, 40: Cosmosiin, 41: Quercitrin, 42: Astragalin, 43: Nicotiflorin, 47: Quercetin, 48: Naringenin, 49: Hesperetin, 50: Luteolin, 52: Kaempferol, 53: Apigenin, 55: Chrysin, and 56: Acacetin. (B). Chromatogram of propolis ethanol extract compounds. (Karagecili et al., 2023)
Customer Testimonials
-
“We used Creative BioMart’s assays to evaluate CYP3A4, CYP2D6, and CYP2C9 inhibition for a lead oncology compound. The results were delivered quickly, formatted for direct regulatory submission, and confirmed by our internal follow-up. Their ability to customize the assay conditions to match our compound's characteristics made a real difference.”
— Director of Pharmacology | Biotech Startup
-
“We needed to characterize Phase I metabolic stability for several NCEs across human and rat liver microsomes. Creative BioMart not only provided robust, reproducible data but also offered insights on metabolite identification that helped steer our med-chem team in a better direction.”
— Senior Scientist, Drug Discovery Unit | Global Pharma
-
“For a study on natural compound metabolism, we used Creative BioMart’s hepatocyte-based assay system to assess glucuronidation and sulfation pathways. Data quality matched our in-house controls, and their analysis included clear kinetic modeling.”
— Principal Investigator | Academic Medical Center
-
“Creative BioMart’s metabolite profiling and LC-MS/MS expertise helped pinpoint an unexpected reactive intermediate in our anti-inflammatory drug candidate. Their guidance helped us de-risk the molecule and avoid costly late-stage surprises.”
— R&D Project Lead | Specialty Pharma
FAQs
-
Q: What types of metabolism assays do you offer?
A: We provide in vitro assays including CYP450 inhibition, metabolic stability (microsomes/hepatocytes), metabolite profiling, and drug-drug interaction studies. We also offer binding assays, species comparison, and physicochemical property assessments. -
Q: Can your assays be customized for early discovery or regulatory submission stages?
A: Yes! Our assays are highly flexible—whether you're screening large libraries in discovery or need GLP-style data for regulatory filing, we adapt accordingly. -
Q: How much sample is required to run a full metabolism panel?
A: Typically, 1–5 mg of compound is sufficient, but we’ll advise based on your selected services and detection method. -
Q: What species do you support for cross-species metabolism studies?
A: We offer human, rat, mouse, dog, monkey, and other species upon request—ideal for bridging preclinical and clinical development. -
Q: How do you ensure data quality and reproducibility?
A: Our assays use validated protocols, internal standards, and are run on high-end LC-MS/MS platforms. Each report includes detailed QC metrics. -
Q: Can I integrate metabolism data with other services you offer?
A: We use standardized protocols, triplicate runs, and internal controls to ensure consistency. Our dual-luciferase setups allow for normalization, and we include raw data, QC metrics, and interpretation notes in every report. -
Q: Will I receive help interpreting the data?
A: Yes! All projects include a complimentary consultation with one of our metabolism experts to help you make sense of your results.
Resources
Related Services
- Drug Discovery Screening
- Drug Analysis Service
- High-Throughput Screening
- Protein Pathway Profiling
- Ion Channel Screening Assays
- Protein-Lipids/Nucleic Acid Interaction Assay and Screening
Related Products
References:
- Kabir M, Padilha EC, Shah P, et al. Identification of selective cyp3a7 and cyp3a4 substrates and inhibitors using a high-throughput screening platform. Front Pharmacol. 2022;13:899536. doi:10.3389/fphar.2022.899536
- Karagecili H, Yılmaz MA, Ertürk A, et al. Comprehensive metabolite profiling of Berdav propolis using LC-MS/MS: determination of antioxidant, anticholinergic, antiglaucoma, and antidiabetic effects. Molecules. 2023;28(4):1739. doi:10.3390/molecules28041739
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

