cAMP Accumulation Assay
The cAMP Accumulation Assay is a robust, cell-based functional assay designed to measure cyclic adenosine monophosphate (cAMP), a critical second messenger in G protein-coupled receptor (GPCR) signaling. At Creative BioMart, we provide highly sensitive and reproducible assay services for evaluating GPCR activity and related cellular processes. With flexible assay formats and miniaturization capabilities, our platform is tailored for both high-throughput screening and detailed functional studies. This service enables precise characterization of agonists, antagonists, inverse agonists, allosteric modulators, and enzyme activities relevant to the cAMP signaling pathway, supporting both academic research and pharmaceutical development.
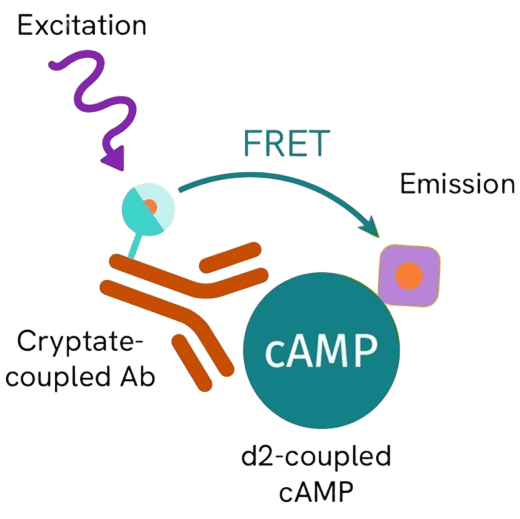
Background: Understanding cAMP Accumulation and GPCR Signaling
G protein-coupled receptors (GPCRs) constitute one of the largest and most diverse receptor families, mediating numerous physiological processes and serving as key drug targets. GPCR activation primarily signals through two main intracellular pathways: the cyclic AMP (cAMP) pathway and the phosphatidylinositol pathway. The cAMP pathway is particularly important for understanding Gs and Gi protein-coupled receptor functions. Measuring intracellular cAMP levels provides valuable insights into receptor activation, signaling strength, and pharmacological modulation. Given their central role in drug discovery, reliable cAMP accumulation assays are indispensable tools for pharmacological screening and mechanism-of-action studies.
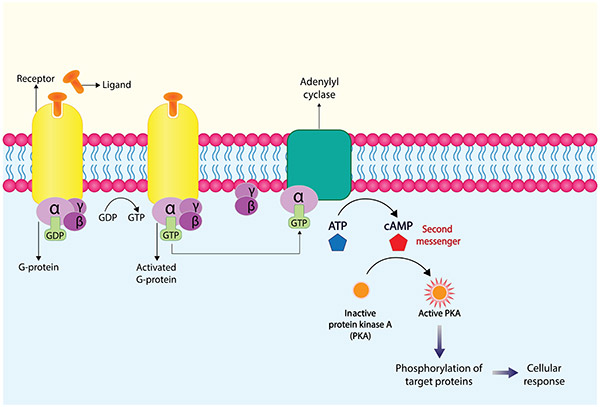
Figure 1. GPCR signaling pathway.
Our cAMP Accumulation Assay Services
Creative BioMart’s cAMP Accumulation Assay service offers a versatile and optimized platform for detecting and quantifying cAMP levels in a cell-based system.
Service Workflow

Service Highlights
|
Features |
Details |
|---|---|
|
Assay Type |
Cell-based functional assay for second messenger quantification. |
|
Detection Principle |
Competitive displacement of biotinylated cAMP probe with intracellular cAMP levels. |
|
Sensitivity & Reliability |
Designed for sensitivity adjustment across a wide concentration range, ensuring consistent and accurate results under diverse experimental conditions. |
|
Flexible Formats |
Available in 96-, 384- or 1536-well formats, supporting both small-scale exploratory studies and large-scale high-throughput screening campaigns. |
|
Sample Versatility |
Compatible with both fresh and frozen cells, offering flexibility for varied research workflows and project timelines. |
|
Miniaturization Options |
Assay formats of 100 μL, 20 μL, and down to < 10 μL for resource-efficient testing. |
|
Applications |
|
Detection Principle of cAMP Accumulation Assay
The assay measures intracellular cAMP levels using a competitive binding strategy. Endogenous cAMP produced by cells competes with a biotinylated cAMP probe that interacts with streptavidin-donor and anti-cAMP–acceptor beads. When the beads are in close proximity, a detectable signal is generated. Activation of Gs-coupled GPCRs by an agonist increases intracellular cAMP, displacing the biotinylated probe and reducing the signal in a proportional manner. Antagonist and inverse agonist effects can be quantified using the same principle. For Gi-coupled receptors, cAMP changes are measured after pre-stimulation with forskolin.
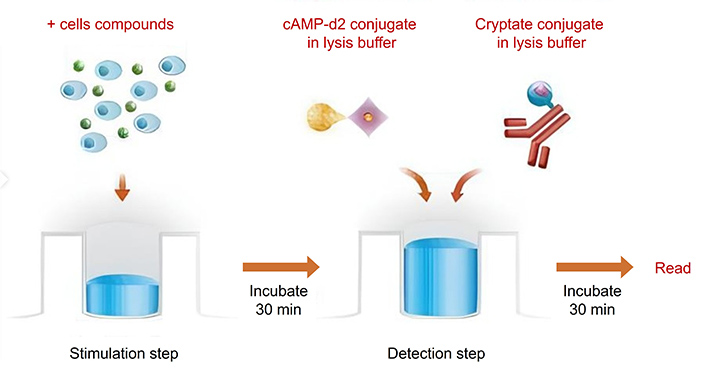
Advantages of Choosing Creative BioMart for cAMP Assays
- High Sensitivity & Specificity: Optimized detection range for accurate cAMP measurement.
- Flexible Formats: Suitable for small-scale studies and high-throughput screening.
- Comprehensive Applications: Covers GPCR pharmacology, enzyme activity, and drug screening.
- Customizable Workflow: Tailored assay design to match specific client research goals.
- Proven Reliability: ISO-standard laboratory practices ensure reproducible results.
- Expert Support: Experienced team providing data interpretation and technical consultation.
Case Studies in cAMP Accumulation and GPCR Research
Case 1: cAMP Signaling and the Lumit Assay
Mikheil et al., 2024. doi:10.1038/s41598-024-55038-0
Synthesized by adenylyl cyclase and degraded by phosphodiesterases, cAMP signaling is tightly controlled, and its dysregulation is linked to cardiovascular, neurological, metabolic, and oncological disorders. Given its clinical relevance, cAMP-targeted therapies are under active investigation. Here, the researchers present the cAMP Lumit assay, a highly specific bioluminescent platform with a wide dynamic range and large assay window. Its homogeneous, high-throughput format offers an efficient tool for drug discovery programs targeting cAMP-related pathways.
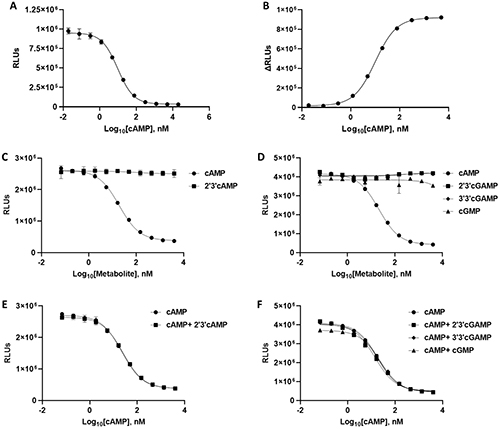
Figure 2. cAMP Lumit Assay range and specificity. (A,B) cAMP standard curve (n = 3 ± SD). (C,D) Titration of other metabolites in comparison with cAMP titration in cAMP Lumit assay (n = 2 ± SD). (E,F) 10 µM of potential interfering metabolites (or buffer) were included in a cAMP titration assay (n = 2 ± SD). (Mikheil et al., 2024)
Case 2: Partial agonism of TRV130 and PZM21 at μ-receptors
Singleton et al., 2021. doi:10.1111/bph.15409
This study investigated the efficacy of TRV130, PZM21, morphine, and DAMGO in β-arrestin2 recruitment and cAMP inhibition using CHO cells expressing μ-receptors. While morphine, PZM21, and TRV130 behaved as partial agonists in β-arrestin2 recruitment, only TRV130 showed partial agonism in the cAMP assay. Reducing receptor availability with β-FNA further highlighted partial agonism in TRV130, morphine, and PZM21. In vivo, TRV130 produced potent anti-nociception in wild-type mice without tolerance, but tolerance emerged in μ+/− mice by day 4. These results stress the critical role of receptor reserve in defining μ-receptor agonist activity and tolerance potential.
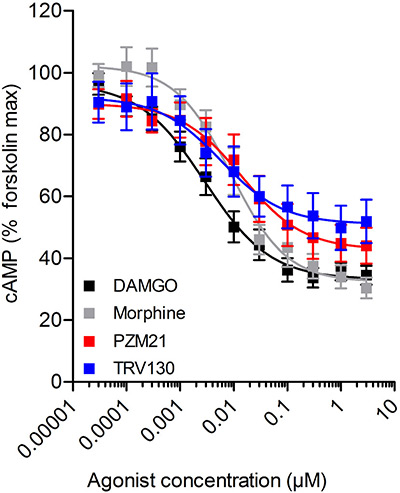
Figure 3. μ-Receptor activation inhibits cAMP accumulation. Concentration–response relationships of DAMGO, morphine, PZM21 and TRV130 as inhibitors of cAMP accumulation in CHO cells transiently expressing the pGloSensor-22F protein. (Singleton et al., 2021)
Customer Testimonials for cAMP Assay Services
“We engaged Creative BioMart for a large-scale screening campaign targeting Gs-coupled GPCRs. Their cAMP Accumulation Assay in a 384-well format allowed us to screen more than 10,000 compounds efficiently. The sensitivity adjustment across different cAMP ranges ensured we didn’t miss subtle hits, and their detailed analysis reports helped us prioritize lead candidates quickly. Their ability to balance scale, speed, and accuracy made them a reliable partner in our discovery pipeline.”
— Senior Director of Pharmacology | Global Pharmaceutical Company
“Our lab used Creative BioMart’s cAMP Accumulation Assay to investigate Gi-coupled receptor modulation in neuronal cells. Since we work with frozen cell banks to fit academic schedules, their assay’s compatibility with frozen cells was a major advantage. The team’s flexibility and willingness to customize conditions helped us detect both agonist and antagonist activity with high precision.”
— Professor of Molecular Pharmacology | Leading Research University
“We collaborated with Creative BioMart to evaluate the potency of novel phosphodiesterase inhibitors. Their cAMP assay provided precise degradation kinetics, which were crucial for determining IC50 values. The data reproducibility across multiple batches impressed both our scientists and regulatory team. By using their high-throughput 96- and 384-well formats, we advanced our PDE program to preclinical candidate selection three months ahead of schedule.”
— Head of Discovery Biology | Mid-Sized Biotech Company
“As a startup working on first-in-class allosteric modulators, we needed a partner who could adapt assays to our unconventional targets. Creative BioMart delivered a tailored cAMP Accumulation Assay with miniaturized formats that saved precious sample volume. Their technical experts guided us through interpreting inverse agonist activity, providing clarity at a stage where resources were tight. The quality of their work not only advanced our program but also gave our investors’ confidence to support the next funding round.”
— Director of Preclinical Research | Emerging Biotech Startup
FAQs About cAMP Accumulation Assays
-
Q: What makes your cAMP Accumulation Assay different from other providers?
A: Our assay is designed for sensitivity adjustment across a wide concentration range, ensuring accurate results under diverse conditions. We also offer compatibility with multiple formats (96-, 384- or 1536-well) and miniaturization down to <10 μL, making it ideal for both small-scale studies and high-throughput screening campaigns. -
Q: Can I use frozen cells with your assay, or do I need fresh cultures?
A: Both fresh and frozen cells can be used seamlessly in our platform. This flexibility allows researchers to align experiments with their project timelines without compromising assay quality or reproducibility. -
Q: What applications can your cAMP Accumulation Assay support?
A: Our service is widely applicable for studying Gs- or Gi-coupled GPCR activation, screening agonists, antagonists, inverse agonists, and allosteric modulators, monitoring phosphodiesterase (PDE) activity, and assessing adenylate cyclase function. This versatility makes it valuable across early discovery, mechanistic studies, and drug profiling. -
Q: How do you ensure data reliability and consistency across projects?
A: We operate under ISO13485 quality management standards and apply strict inter-batch controls. This ensures that results are reproducible across experiments, which is especially critical for regulated research or long-term drug development programs. -
Q: Can the assay be customized for my specific project needs?
A: Absolutely. Our team works closely with each client to optimize assay conditions, including receptor type, cell source, assay format, and detection parameters. We also provide tailored data analysis and reporting to support both academic publications and pharmaceutical development. -
Q: How fast is the turnaround time for results?
A: Turnaround time depends on project complexity and scale, but our streamlined workflows and high-throughput capabilities allow us to deliver results efficiently. For standard assays, preliminary data can often be provided within 2–4 weeks after project initiation.
Resources
Related Services
Related Products
References:
- Mikheil D, Larsen MA, Hsiao K, et al. A bioluminescent and homogeneous assay for monitoring GPCR-mediated cAMP modulation and PDE activity. Sci Rep. 2024;14(1):4440. doi:10.1038/s41598-024-55038-0
- Singleton S, Baptista‐Hon DT, Edelsten E, McCaughey KS, Camplisson E, Hales TG. TRV130 partial agonism and capacity to induce anti‐nociceptive tolerance revealed through reducing available μ‐opioid receptor number. British J Pharmacology. 2021;178(8):1855-1868. doi:10.1111/bph.15409
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

