MSK
Related Symbol Search List
Immunology Background
About MSK
Mitogen- and stress-activated kinase (MSK) is a class of protein kinases belonging to the AGC (Protein Kinase A/G/C-like) family. MSK has been extensively studied and is of interest because of its important role in cell signaling pathways.
MSK consists of two main members: MSK1 and MSK2. They are named after their activation in response to a variety of stimulatory signals (e.g., growth factors, stress, inflammatory factors, etc.). MSK1 and MSK2 are structurally similar, but may differ in some respects.
Activation of MSK kinases usually involves a multistage kinase cascade reaction. A common activation mechanism is through members of the MAPK (Mitogen-activated protein kinase) family, such as ERK (Extracellular signal-regulated kinase), p38, and JNK (c-Jun N-terminal kinase). These kinases can be activated by external stimulatory signals, which then phosphorylate and activate MSK. Activated MSK further phosphorylates downstream substrates, such as transcription factors and histones, to regulate gene expression and cell biological responses.
Studies have shown that mitogen- and stress-activated kinase 1 (MSK1) is activated by either ERKs or p38 MAP kinases in response to stress or mitogenic extracellular stimuli. MSK1 is involved in the transcriptional activation of genes, including the inflammatory gene interleukin-6 and immediate early response genes such as c-fos, c-phosphate, and c-phosphate. MSK1 is involved in the transcriptional activation of genes, including the inflammatory gene interleukin-6 and immediate early response genes such as c-fos, c-jun, mkp-1 (MAPK phosphatase -1), and nurr1. MSK1 has also been reported to phosphorylate chromatin proteins histone H3 and HMG-14. Further, MSK1 phosphorylates various transcription factors including cAMP-response element-binding protein (CREB), activating transcription factor 1 (ATF1) and the p65 subunit of NF-κB. Recently, MSK1 was shown to play a positive role in the control of cell proliferation of HaCaT keratinocytes and might play a role in pathogenesis of psoriasis by regulating the production of pro-inflammatory cytokines in psoriatic epidermis. MSK1 was suggested to be activated in response to tumor promoters such as EGF, TPA or UVB.
Mechanism of Action of MSK
The mechanism of action of Mitogen- and stress-activated kinase (MSK) mainly involves its phosphorylation of downstream substrates to regulate gene expression and cellular functions. The following are the main mechanisms of MSK action:
- Phosphorylation of Transcription Factors
MSK can phosphorylate several transcription factors, including CREB (cAMP Response Element-Binding protein), ATF1 (Activating Transcription Factor 1), NF-κB (Nuclear Factor-κB), and so on. Phosphorylated transcription factors are able to change their activity and DNA binding ability, thus affecting the transcription of specific genes. These genes may be involved in cell proliferation, inflammatory response, immune response and other important biological processes.
- Phosphorylation of Histones
MSK can also phosphorylate histone H3, particularly at the serine 10 site. This phosphorylation modification can change the structure and accessibility of chromatin, thus affecting the transcriptional regulation of genes.MSK-mediated histone phosphorylation plays an important role in the regulation of gene expression.
- Downstream Signaling
Phosphorylation events of MSK kinase activity can trigger downstream signaling pathways. Phosphorylated substrates can activate or inhibit other protein molecules, forming a signaling cascade response that in turn regulates the biological response of the cell. For example, phosphorylation of MSK kinase activity can activate MAPK family members, such as ERK, to further regulate processes such as cell proliferation and survival.
- Gene Expression Regulation
By phosphorylating transcription factors and histones, MSK kinases can affect the expression levels of specific genes. This may involve directly altering the activity of transcription factors, DNA binding affinity and transcriptional activation capacity, or indirectly affecting gene transcription by altering chromatin structure. This mechanism of gene expression regulation allows MSK to play an important role in biological processes such as cell proliferation, inflammatory responses, and immune responses.
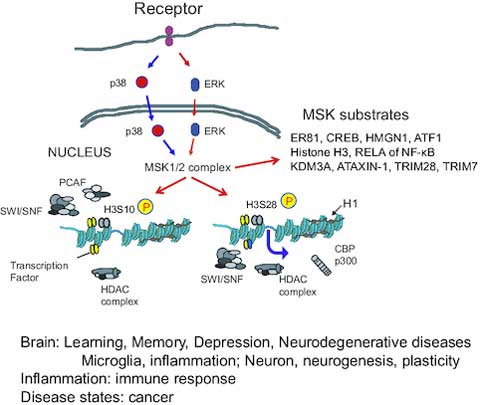 Fig.1 A schematic model showing the role of MSK1 in cigarette smoke-induced NF-kB signaling and chromatin modifications. (Sundar I K, et al., 2012)
Fig.1 A schematic model showing the role of MSK1 in cigarette smoke-induced NF-kB signaling and chromatin modifications. (Sundar I K, et al., 2012)
Functions of MSK
MSK activation directly affects cell fate and physiological processes. It plays an important role in cell proliferation, survival and differentiation. By regulating gene transcription, MSK is involved in important physiological processes such as cell cycle regulation, apoptosis and inflammation. In addition, MSK is closely associated with the onset and development of many diseases, including inflammatory diseases, tumors and neurodegenerative diseases.
- Cell Proliferation and Survival
By phosphorylating transcription factors and histones, MSK regulates the expression of genes associated with cell proliferation and survival. In addition, active phosphorylation of MSK activates downstream signaling pathways, such as members of the MAPK family, which are further involved in cell proliferation and survival signaling.
- Inflammatory Response and Immune Response
Inflammatory factors and immune stimuli can activate MSK, thus triggering the inflammatory response and activation of immune cells. phosphorylation of MSK regulates the expression of inflammation-related genes, such as inflammatory mediators and cytokines, and thus regulates the process of the inflammatory response and immune response.
- Nervous System Functions
MSK is involved in the regulation of neuronal development, survival and plasticity, etc. Phosphorylation of MSK activity can affect neuronal gene expression and neurotransmitter release, thus participating in the regulation of nervous system function.
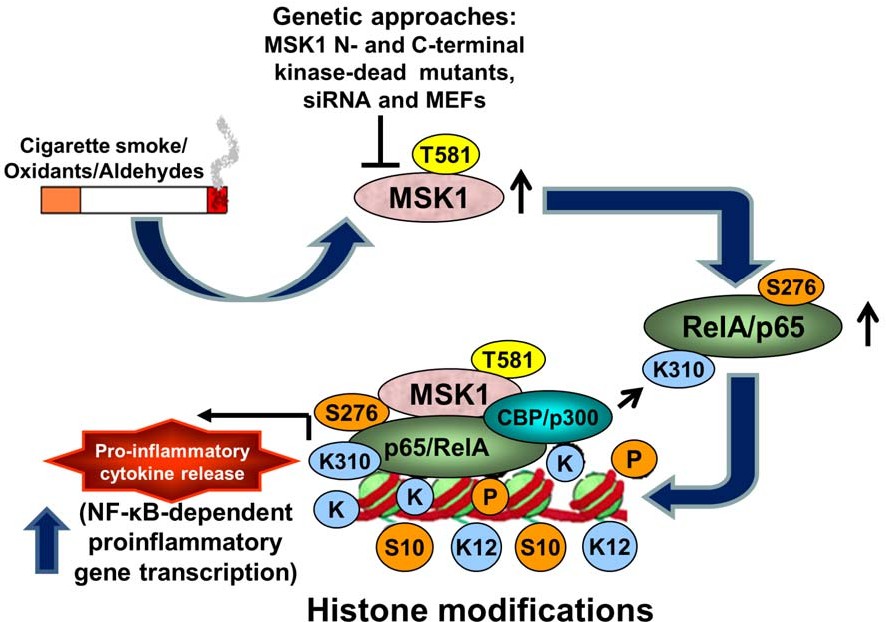 Fig.2 The mitogen- and stress-activated protein kinases (MSK) are epigenetic modifiers that regulate gene expression in normal and disease cell states. (Sattarifard H, et al., 2023)
Fig.2 The mitogen- and stress-activated protein kinases (MSK) are epigenetic modifiers that regulate gene expression in normal and disease cell states. (Sattarifard H, et al., 2023)
Available Resources for MSK
The active phosphorylation events of MSK can trigger downstream signaling pathways and affect cellular biological responses, which are involved in transcriptional regulation, histone modification, cell proliferation and survival, inflammatory response, immune response, and the regulation of nervous system functions.The functional diversity of MSK makes it important in cell biology and disease genesis. Creative BioMart offers a variety of MSK-related research products, such as recombinant proteins, cell and tissue lysates, and protein pre-coupled magnetic beads, as well as customizable services and other resources to support your research in the field of MSK. The following MSK is displayed, click to view all related molecules/targets and research reagents. For further information or to purchase products, please contact us. We are committed to providing the highest quality resources and support for your research to help you succeed.
References:
- Sundar I K, Chung S, Hwang J, et al. Mitogen-and stress-activated kinase 1 (MSK1) regulates cigarette smoke-induced histone modifications on NF-κB-dependent genes[J]. PloS one, 2012, 7(2): e31378.
- Sattarifard H, Safaei A, Khazeeva E, et al. Mitogen-and stress-activated protein kinase (MSK1/2) regulated gene expression in normal and disease states[J]. Biochemistry and Cell Biology, 2023, 101(3): 204-219.
- Kim HG, Lee KW, Cho YY, et al. Mitogen- and stress-activated kinase 1-mediated histone H3 phosphorylation is crucial for cell transformation. Cancer Res. 2008;68(7):2538-2547.

