Biomarker Service
Background
A biomarker is a measurable indicator of a biological state or condition, used to assess health, disease, physiological processes, or responses to treatments. Biomarkers can be molecules (e.g., proteins, DNA), cells, imaging features, or physiological characteristics (e.g., blood pressure) that provide objective insights into normal or pathological processes.
Biomarker services encompass a suite of technologies and analytical approaches designed to discover, validate, and apply biological markers (biomarkers) for disease diagnosis, prognosis, therapeutic monitoring, and personalized treatment. These services integrate multidisciplinary fields such as genomics, proteomics, metabolomics, and bioinformatics, driven by advancements in high-throughput technologies like next-generation sequencing (NGS), mass spectrometry, and liquid biopsy platforms.
Types of Biomarkers
- Diagnostic Biomarkers: Identify the presence of a disease (e.g., PSA for prostate cancer, troponin for heart damage).
- Prognostic Biomarkers: Predict disease progression or outcome (e.g., KRAS mutations in colorectal cancer indicate poor prognosis).
- Predictive Biomarkers: Guide treatment selection by indicating likely response to therapy (e.g., HER2 overexpression in breast cancer predicts response to trastuzumab).
- Monitoring Biomarkers: Track disease status or treatment efficacy (e.g., HbA1c for diabetes management, ctDNA for cancer recurrence).
- Pharmacodynamic Biomarkers: Measure the biological effect of a drug (e.g., reduced amyloid-beta levels in Alzheimer’s trials).
Applications in Drug Development
- Early Discovery: Biomarker services help identify novel drug targets and potential therapeutic candidates by analyzing biological samples for specific markers that indicate disease progression or treatment response.
- Preclinical and Clinical Trials: These services are essential for validating biomarkers as endpoints in clinical trials, ensuring that the selected markers accurately reflect the efficacy and safety of a drug.
- Patient Stratification: Biomarkers are used to classify patients based on their likelihood of responding to a particular treatment, thereby improving clinical trial outcomes and reducing costs.
Figure 1. Applications of biomarkers. (Challa et al., 2020)
Creative BioMart supports your drug development needs by providing a full suite of Biomarker Assay Development and Validation Services as well as biomarker testing services (both large and small scale) across the drug development continuum, from early proof concept to commercial laboratory testing. With over 15 years of experience and industry leadership in developing and performing immunoassays on human and animal specimens, our biomarker analysis can accelerate your drug discovery and development programs.
What We Offer?
Service Details
Custom Assay Development and Validation
We offer comprehensive custom assay development and validation services tailored to specific biomarker targets and study objectives. Our team works closely with clients to design assays optimized for sensitivity, specificity, and reproducibility. Validation is performed in alignment with relevant regulatory and scientific standards to ensure data robustness and reliability for preclinical research applications.
Biomarker Immunoassays
Our biomarker immunoassay services include both single and multiplex formats to quantitatively measure proteins such as cytokines, chemokines, growth factors, and other analytes. Utilizing platforms including ELISA, MSD, and Luminex, we support a wide range of sample types and throughput needs. Assays can be developed de novo or adapted from existing protocols, depending on study requirements.
Genomics Analysis
Our genomics analysis capabilities provide comprehensive support for DNA and RNA-based investigations, including gene expression profiling, SNP genotyping, and transcriptome analysis. Utilizing technologies such as qPCR, microarrays, and next-generation sequencing (NGS), we generate high-quality genomic data to support biomarker discovery, mechanism-of-action studies, and target validation.
Proteomics
We deliver proteomics services for quantitative and qualitative protein analysis, leveraging techniques such as mass spectrometry (LC-MS/MS) and multiplex immunoassays. Applications include biomarker discovery, pathway analysis, and protein profiling in preclinical models. Our workflows are optimized for various biological matrices and are designed to meet rigorous standards for reproducibility and analytical accuracy.
Sample Analysis on Diverse Platforms
Our team provides sample analysis across multiple platforms to support a broad spectrum of biomarker assays. Available technologies include MSD, Luminex, LC-MS, flow cytometry, and qPCR, among others. We select the most appropriate analytical platform based on the specific biomarker, matrix, and project goals, ensuring high data quality and efficient turnaround times.
Service Procedure
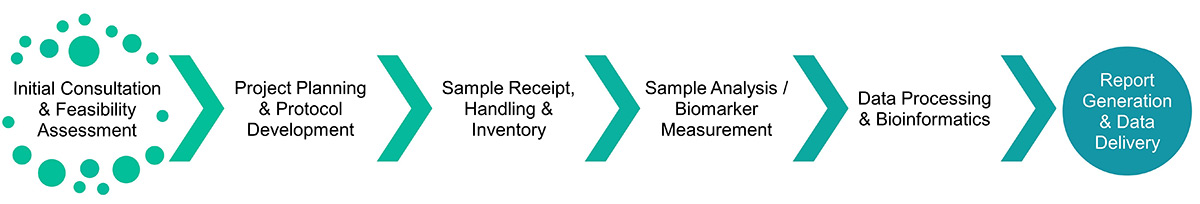
Technologies & Methods
Creative BioMart is equipped with a series of biochemical instruments and various tools to support nonclinical biomarker research across molecular, proteomic, and immunological domains. For immunoassay-based biomarker quantification, we utilize ELISA, Luminex xMAP, and Meso Scale Discovery (MSD) platforms, enabling sensitive and multiplexed detection of proteins such as cytokines, chemokines, and growth factors. For genomic and molecular analysis, we operate quantitative PCR (qPCR) and digital PCR systems for precise gene expression and copy number analysis. We also employ microarray platforms and next-generation sequencing (NGS) technologies for transcriptome-wide profiling and targeted gene analysis. Our proteomics capabilities are supported by liquid chromatography–tandem mass spectrometry (LC-MS/MS) systems for high-resolution protein identification and quantification. We also conduct multiplex protein profiling for high-throughput applications, along with post-translational modification (PTM) analysis to capture functional protein changes.




Why Choose Us?
- Efficiency and Scalability: Projects are executed with agile timelines and a scalable operational infrastructure, enabling efficient handling of studies of varying scope and complexity.
- Commitment to Compliance: All services are delivered in strict accordance with contracted study requirements, ensuring transparency, consistency, and adherence to regulatory and client expectations.
- Rigorous Quality Assurance: All study activities are conducted under a stringent quality assurance framework, encompassing performance assessments, control evaluations, standard operating procedures, equipment validation, and personnel qualifications.
- Timely Results and Responsive Support: Rapid data delivery is complemented by dedicated and responsive customer service, facilitating timely decision-making and client satisfaction.
- Data Integrity and Security: Advanced data management systems and rigorous quality control measures are employed to safeguard data accuracy, integrity, and confidentiality throughout the study lifecycle.
- Multi-Platform Sample Analysis: Integration of diverse analytical technologies based on biomarker type and sample matrix, supporting for various biological specimens including plasma, serum, tissue, and cell lysates.
Case Study
Case 1: Analysis of urinary TGF-β1, MCP-1, NGAL, and IL-17 as biomarkers for lupus nephritis
Susianti et al., 2015. doi:10.1016/j.pathophys.2014.12.003
This study evaluated the diagnostic value of four urinary biomarkers—TGF-β1, MCP-1, NGAL, and IL-17—in patients with lupus nephritis (LN). Among 70 participants (38 with severe LN, 12 with mild LN, and 20 healthy controls), levels of uMCP-1 and uNGAL were significantly elevated in both mild and severe LN groups, while uTGF-β1 and uIL-17 were significantly higher only in severe cases. Diagnostic performance was assessed via AUC: uNGAL and uMCP-1 had the highest diagnostic accuracy, while uIL-17 and uTGF-β1 showed moderate performance.
Notably, uNGAL correlated significantly with disease activity and chronicity indices, making it the most promising standalone biomarker. The best-performing biomarker combination was uTGF-β1 + uNGAL, followed by uMCP-1 + uNGAL, offering improved sensitivity and specificity for LN detection.
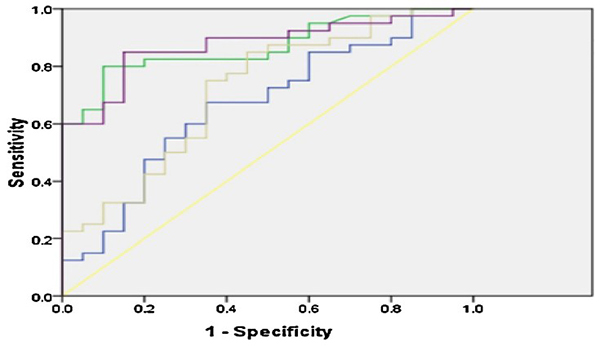
Figure 2. ROC curve on uTGF-β1, uMCP-1, uNGAL and uIL-17. Yellow line: reference line; blue line: urinary transforming growth factor beta 1; green line: urinary monocyte chemoattractant protein 1; purple line: urinary neutrophil gelatinase-associated lipocalin; gray line: urinary interleukin-17. (Susianti et al., 2015)
Case 2: DNA methylation as an epigenetic biomarker
Zwartkruis et al., 2025. doi:10.1016/j.isci.2025.112461
This study investigated DNA methylation across the SMN2 gene in patients with spinal muscular atrophy (SMA) to explore potential biomarkers beyond SMN2 copy number, which only partially explains disease severity and treatment response. Using long-read nanopore and short-read bisulfite sequencing, researchers analyzed the methylation landscape in 29 SMA patients and a broader cohort of 365 blood-derived samples. While they found tissue-specific and intronic methylation patterns, there was no correlation between blood-derived SMN2 methylation and disease severity or treatment response, ruling it out as a predictive biomarker. However, they did observe age-related methylation changes in intron 1 and the 3′UTR, suggesting a developmental or aging-related regulatory role. Overall, the study highlights the complexity of epigenetic regulation in SMA and offers a methodological framework for methylation profiling in large, repetitive genes.
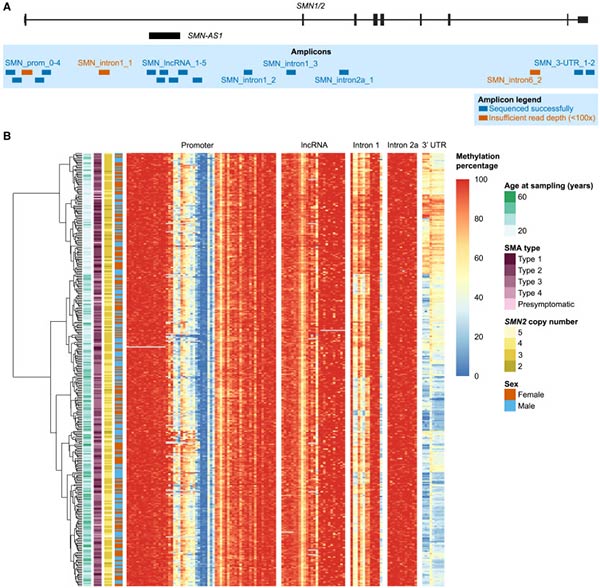
Figure 3. DNA methylation in the SMN2 gene can be determined by targeted bisulfite sequencing. (A) Location of 18 amplicons, 15 of which were sequenced successfully (a total of ∼6.6 kb around SMN2). (B) Heatmap of DNA methylation percentage per CpG site determined with targeted bisulfite sequencing. (Zwartkruis et al., 2025)
Customer Testimonials
-
"We needed high-throughput cytokine profiling for our lead compound targeting rheumatoid arthritis. Creative BioMart delivered not only a robust multiplex assay but also helped us interpret subtle changes in IL-17 and IFN-γ levels that correlated with patient response. Their ability to fine-tune the assay to our needs saved us weeks of optimization."
— Senior Scientist | Biotech Startup Focused on Autoimmune Therapies
-
"Our lab worked with Creative BioMart to identify predictive biomarkers for colorectal cancer immunotherapy response. Their protein expression microarray platform allowed us to profile over 1,000 markers simultaneously. The data clarity and reproducibility were excellent, and the team supported our downstream bioinformatics analysis. We're now planning a follow-up study based on the leads we discovered."
— Principal Investigator | University Cancer Research Center
-
"We engaged Creative BioMart for epigenetic biomarker analysis during our target validation process. Their custom assay development using DNA methylation and histone modification markers helped us confirm tissue-specific expression patterns. Their reports were well-documented, and regulatory-friendly—exactly what we needed for IND submission support."
— Head of Preclinical Development | Mid-size Pharm
-
"When developing our new blood-based diagnostic test for early lung cancer, we turned to Creative BioMart for their biomarker discovery expertise. Their codon optimization and expression profiling services helped us accelerate our prototype development. They’re fast, flexible, and truly understand the needs of a diagnostics company under tight timelines."
— CEO | Diagnostics Company
FAQs
-
Q: What types of biomarkers do you specialize in?
A: We work with protein, DNA/RNA, metabolite, lipid, and cellular biomarkers across multiple therapeutic areas including oncology, neurology, inflammation, and infectious disease. -
Q: How accurate and reliable are your biomarker tests?
A: Our state-of-the-art technologies and stringent quality controls ensure high accuracy and reliability, with validation rates exceeding 98%. -
Q: How fast can you deliver results?
A: Depending on complexity, initial data delivery can be as fast as 4–6 weeks from project initiation. We pride ourselves on speed and scientific accuracy. -
Q: Do you offer custom assay development?
A: Yes! We love building from the ground up. Our team will work with you to define targets, optimize protocols, and validate to your specifications. -
Q: Can you support biomarker discovery and validation?
A: Absolutely, we offer integrated services for biomarker discovery, validation, and clinical application development.
Resources
Related Services
- Cancer Biomarkers
- Protein Pathway Profiling
- Cytokine and Receptor Analysis
- Epigenetics-Based Assay Development
Related Products
References:
- Challa S, Sri-Tirumala-Peddiniti RCPK, Neelapu NRR. Development and evaluation of biomarkers for early detection of cancer. In: Veera Bramhachari P, Neelapu NRR, eds. Recent Advancements in Biomarkers and Early Detection of Gastrointestinal Cancers. Springer Singapore; 2020:27-43. doi:10.1007/978-981-15-4431-6_3
- Susianti H, Iriane VM, Dharmanata S, et al. Analysis of urinary TGF-β1, MCP-1, NGAL, and IL-17 as biomarkers for lupus nephritis. Pathophysiology. 2015;22(1):65-71. doi:10.1016/j.pathophys.2014.12.003
- Zwartkruis MM, Kortooms JV, Gommers D, et al. Comprehensive analysis across SMN2 excludes DNA methylation as an epigenetic biomarker for spinal muscular atrophy. iScience. 2025;28(5):112461. doi:10.1016/j.isci.2025.112461
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

