Pharmaceutical Stability Analysis
Background on Pharmaceutical Stability Studies in Drug Development
Pharmaceutical stability analysis is a critical component in the development, manufacturing, and quality control of drug products. It involves the systematic evaluation of a drug’s physical, chemical, microbiological, and biological properties over time under various environmental conditions such as temperature, humidity, and light exposure.
The primary goal of stability analysis is to determine the shelf life and storage conditions that ensure a drug product maintains its safety, efficacy, and quality throughout its intended lifespan. Stability studies provide essential data to support regulatory submissions and labeling claims regarding expiration dates and handling instructions.
Stability testing encompasses a range of assays including potency, degradation product profiling, physical characterization, and microbial integrity assessment. The studies are conducted following international guidelines such as ICH Q1A (R2), USP <1211>, and FDA regulations to guarantee consistency and compliance.
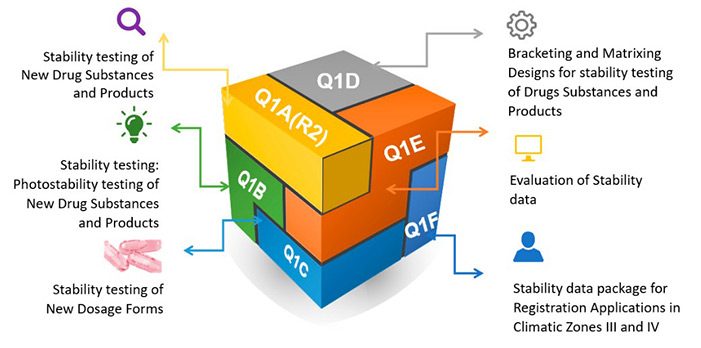
Figure 1. ICH codes and titles. (González-González et al., 2022)
By rigorously monitoring drug stability, manufacturers can detect changes that may impact therapeutic performance, ensuring patients receive safe and effective medications. Creative BioMart provides comprehensive Pharmaceutical Stability Analysis Services covering the full spectrum of ICH-recommended stability conditions and cGMP test methods. Our expertise supports your product’s lifecycle from early research and development to commercial production and post-approval monitoring.
Through rigorous testing under controlled temperature, humidity, photostability, freeze/thaw cycles, and mechanical stress, we generate essential data to characterize your drug’s stability profile. Tailored storage and testing protocols align with your unique requirements and timelines, ensuring flexibility and reliability.
Our Stability Analysis Services for Pharmaceuticals and Biologics
Creative BioMart offers end-to-end stability testing solutions fully integrated within our advanced laboratory complex. Our service ensures your pharmaceutical product meets stringent regulatory requirements and withstands real-world handling and storage conditions.
Service Procedure

Service Items
- ICH guideline-compliant temperature and humidity conditions, including:
- 75% RH / 40°C
- 60% RH / 25°C
- Ambient humidity at 25°C, 30°C, 40°C, 50°C, 55°C
- Refrigeration at 5°C
- Freezing at -20°C and -80°C
- Liquid nitrogen storage
- Custom stability conditions tailored to your protocols
- Forced degradation studies
- Freeze/thaw cycle analysis
- Photostability testing under controlled light exposure
- Mechanical stress testing
- Container compatibility assessments
- In-use studies for devices such as IV bags and infusion pumps
- Drug-substance stability testing
- Accelerated and long-term stability studies
Techniques

Our stability testing employs cutting-edge analytical techniques to deliver detailed, reliable data:
- Gel Electrophoresis and Western Blot for protein integrity
- ELISA and Immunoassays for potency and antigenicity
- Isoelectric Focusing and Capillary Electrophoresis for isoform analysis
- Amino Acid Analysis for sequence confirmation
- Chromatography and Mass Spectrometry for degradation product profiling
- Immunochemistry for epitope mapping and antibody characterization
- Dynamic Light Scattering (DLS) for particle sizing and aggregation
- Far- and Near-UV Circular Dichroism Spectroscopy for secondary and tertiary structure
- Fluorescence Spectroscopy for conformational changes
- Differential Scanning Calorimetry (DSC) for thermal stability
Why Choose Our GMP Platform for Stable Cell Line Development
- Regulatory Confidence: Our testing aligns with FDA, EMA, and ICH guidelines, accelerating your regulatory approvals.
- Tailored Testing: Customized stability protocols and reports designed to fit your specific product and submission needs.
- Cost Efficiency: Streamlined workflows and integrated services reduce your overall testing costs and timelines.
- Risk Reduction: Comprehensive stability data minimizes product recalls and compliance risks.
- Rapid Turnaround: Agile project management ensures timely delivery of results, keeping your development on track.
- Expertise & Infrastructure: Experienced scientists and state-of-the-art GMP-compliant labs guarantee quality and reliability.
Case Studies: Real-World Success in Pharmaceutical Stability Analyses
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Photochemical stability of warfarin potassium in powdered pharmaceutical tablets
Horiguchi-Babamoto and Otsuka, 2021. doi:10.3233/BME-201167
This study examined how grinding warfarin potassium (Wf) tablets, commonly done for pediatric and elderly patients, affects their photostability. Using HPLC-UV analysis, it was found that while bulk Wf powder remained relatively stable under light exposure, ground tablets showed significant photodegradation. Photostability varied among four commercial Wf tablets, with ground generic tablets often degrading more than ground brand-name versions. After 28 days of light exposure, Wf content dropped to 69.79% in brand-name tablets and as low as 31.90% in some generics. Chemometric analysis revealed that excipients like light anhydrous silicic acid and povidone negatively impacted light stability, highlighting formulation-dependent effects.
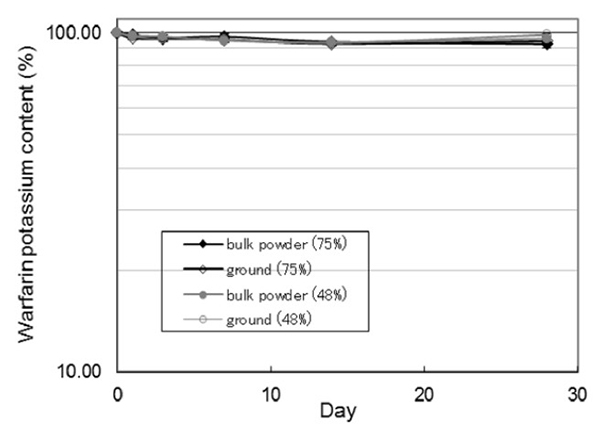
Figure 2. Time course of photodegradation of warfarin potassium content in bulk powder and ground warfarin potassium. (Horiguchi-Babamoto and Otsuka, 2021)
Case 2: Improving telmisartan with hot-melt extruded solid dispersions
Giri et al., 2021. doi:10.1038/s41598-021-99875-9
This study enhanced the solubility and bioavailability of telmisartan (TEL), a poorly water-soluble Class II drug, by developing pH-modulated solid dispersions (TEL pHM-SD) using hot-melt extrusion. Formulations varied in drug, polymer, and sodium carbonate ratios. The optimized formulation (F8) showed a ~30-fold improvement in dissolution and superior stability over commercial tablets. Solid-state analysis confirmed TEL’s transformation to an amorphous state. In vivo studies in rats showed a 6.61-fold increase in Cmax and 5.37-fold increase in AUC compared to TEL powder. These results demonstrate that HME-based pHM solid dispersions offer a promising strategy to enhance hydrophobic drug performance.
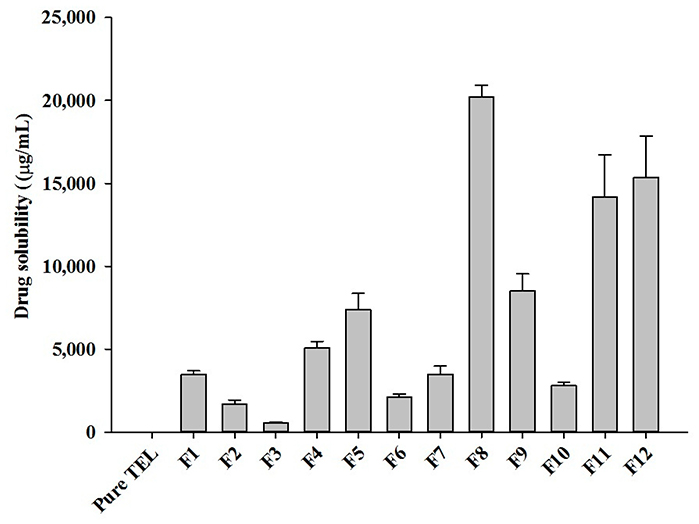
Figure 3. Aqueous solubility of different HME TEL-loaded pH-modulated solid dispersion prepared by varying drug/carrier ratios. (Giri et al., 2021)
What Clients Say About Our Pharmaceutical Stability Testing Services
“Creative BioMart’s stability testing accelerated our biosimilar’s regulatory submission by providing thorough and compliant degradation profiles. Their flexibility with custom conditions was invaluable.”
— Quality Manager | Biosimilar Manufacturer
“The team’s expertise in forced degradation studies helped us understand our protein’s stability under extreme conditions, ensuring robustness of our drug formulation.”
— Senior Scientist | Biotech Firm
“Reliable data and quick turnaround times made Creative BioMart our go-to partner for stability testing during both early development and post-approval stages.”
— R&D Director | Global Pharma Company
“We engaged Creative BioMart for photostability studies on a light-sensitive biologic. Their precise control of light exposure and detailed reporting gave us the confidence to finalize our packaging design.”
— Formulation Scientist | Innovative Biotech Company
FAQs About Our Pharmaceutical Stability Analysis Services
-
Q: What stability conditions do you offer?
A: We cover all ICH-recommended temperature and humidity conditions, including accelerated, long-term, photostability, freeze/thaw cycles, and custom storage environments. -
Q: Can you customize storage and testing protocols?
A: Yes, we tailor storage conditions and testing schedules to meet your specific product requirements and regulatory timelines. -
Q: How do you ensure compliance with regulatory guidelines?
A: All our testing and documentation adhere strictly to cGMP standards and international guidelines such as ICH Q1A (R2), FDA, and EMA requirements. -
Q: What analytical methods do you use for protein stability?
A: We employ a comprehensive suite of techniques, including electrophoresis, chromatography, mass spectrometry, immunoassays, and spectroscopic methods. -
Q: Do you support project management and reporting?
A: Absolutely. We offer full project management, detailed stability reports, and expert consultation to support your submission needs.
Resources
Related Services
Related Products
References:
- Giri BR, Kwon J, Vo AQ, Bhagurkar AM, Bandari S, Kim DW. Hot-melt extruded amorphous solid dispersion for solubility, stability, and bioavailability enhancement of telmisartan. Pharmaceuticals. 2021;14(1):73. doi:10.3390/ph14010073
- González-González O, Ramirez IO, Ramirez BI, et al. Drug stability: ICH versus accelerated predictive stability studies. Pharmaceutics. 2022;14(11):2324. doi:10.3390/pharmaceutics14112324
- Horiguchi-Babamoto E, Otsuka M. Photochemical stability of warfarin potassium in powdered pharmaceutical tablets. BME. 2021;32(2):115-129. doi:10.3233/BME-201167
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

