GPCR Screening Assays
Background
G protein-coupled receptors (GPCRs) form the largest family of drug targets, with 600–1,000 members and over 40% of current pharmaceuticals targeting them. These receptors are central to cell signaling, operating through a trio: the GPCR itself, a G-protein, and a second messenger-generating enzyme. Given their complexity and therapeutic value, GPCRs have long been a focus of drug discovery. While traditional screening focused on agonists and antagonists, current efforts also target inverse agonists and allosteric modulators—molecules that offer more precise control over receptor activity.
Modern GPCR assays aim to unravel these diverse interactions efficiently, enabling the discovery of next-generation therapeutics. From our over 10 years of expertise in the bio-pharmaceutical field, Creative BioMart provides hundreds of functional and radioligand binding GPCR Assays that can be applied for high throughput screening, molecular pharmacology and profiling projects. Our GPCR screening service provides the most comprehensive cell-based assays along with the fastest turnaround time in the industry for rapid efficacy, potency, and selectivity determination. Our highly flexible service allows you to choose the ideal screening assay to meet your project needs in hit identification, selectivity profiling, lead optimization, as well as offers you a cost-effective screening approach to help narrow down your research to individual GPCRs.
What Sets Us Apart
-
10 + Years
Expertise in the biopharmaceutical field
-
100 +
Functional and radioligand binding GPCR assay options
-
24–72 hr
Turnaround for selected high-throughput screens
-
Extensive Applications
High-throughput screening, molecular pharmacology, and profiling projects
-
Project Support Areas
Hit identification, selectivity profiling,
and lead optimization -
Customizable Panels
Target specific GPCRs to match your research
What We Offer?
1. Receptor Binding Assays
-
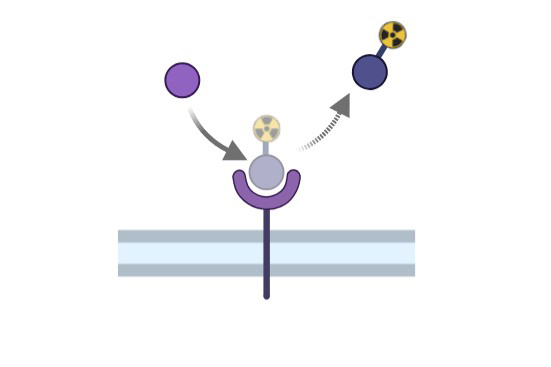
Radioligand Binding Assay
High-throughput; minimal interference; detects agonists and antagonists in one assay
-
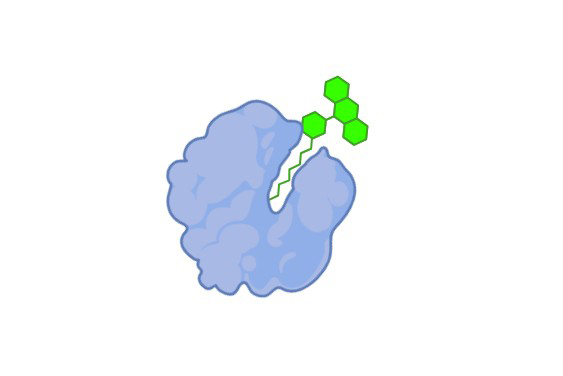
Tagged-Ligand Binding Assay
Non-radioactive; high-throughput; suitable for both agonists and antagonists
2. G-Protein Dependent Assays
-
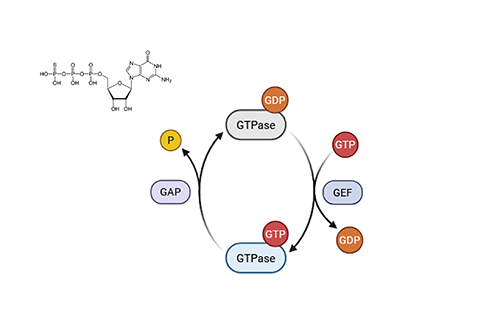
GTPγS Binding
Cell-free assay; distinguishes full/partial agonists, antagonists, inverse agonists, allosteric modulators
-
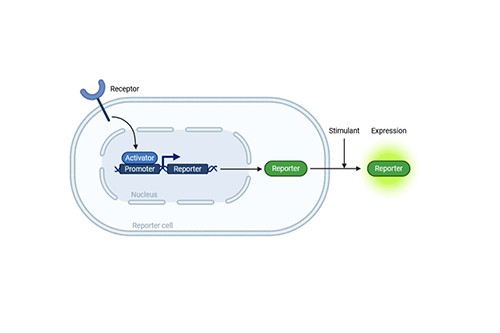
CRE/MRE Reporter Assay
Homogeneous; high-throughput; excellent signal-to-background ratio
-
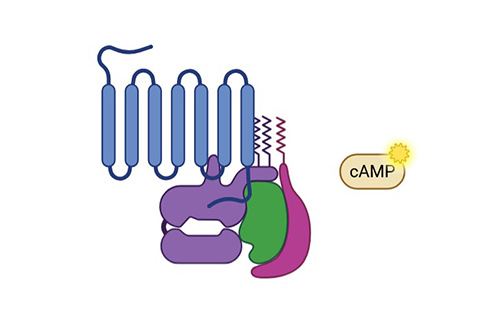
cAMP Assay
Highly sensitive; ideal for large-scale screening
-
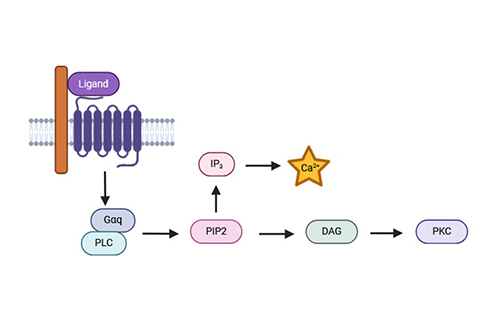
Calcium (Ca2+) Flux Assay
Functional in live cells; detects agonists, antagonists, and allosteric modulators in one assay
-
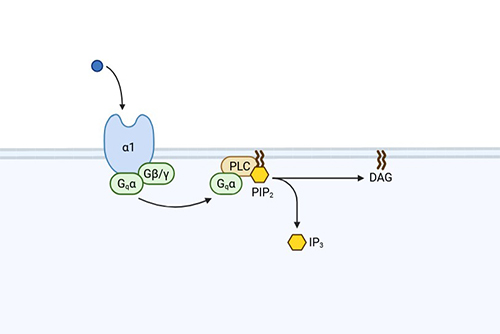
IP1/IP3 Assay
Functional in live cells; suited for slow-binding ligands; high-throughput
3. G-Protein Independent Assays
-
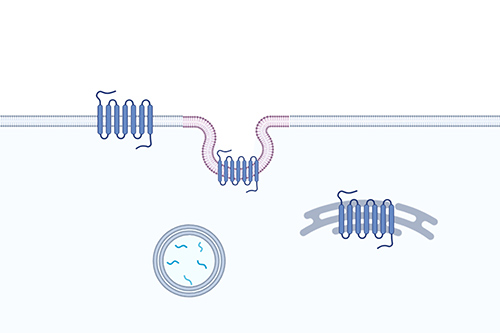
Receptor Trafficking
Image-based, high-content; live-cell compatible; generic across all GPCRs
-
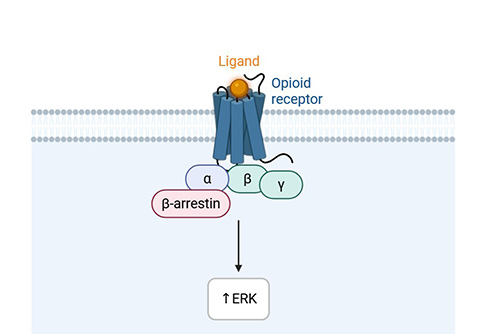
β-Arrestin Recruitment Assay
High-throughput; image or non-image based; live-cell assay; useful for biased signaling
-
Label-Free Assay
Monitors live-cell responses without labels; captures all cellular signaling events
4. Dimerization Assays
-
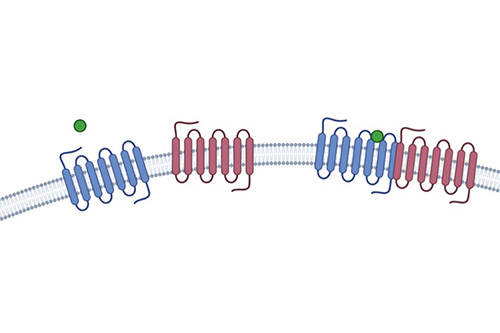
Receptor Dimerization Assay
Targets GPCR heterodimers; emerging pharmacological relevance
Why Choose Us?
- Comprehensive Assay Coverage: From binding and signaling to dimerization, our platform supports full-spectrum GPCR analysis for diverse discovery and profiling needs.
- Biased Signaling Detection: Evaluate both G-protein and β-arrestin pathways to uncover ligand bias, functional selectivity, and nuanced receptor pharmacology.
- Flexible Project Design: We offer fully customizable workflows—from cell models and assay formats to data outputs—to fit your specific scientific and regulatory objectives.
- High Data Quality: All assays are rigorously validated, with strict quality control to ensure reproducibility, sensitivity, and confidence in every data point.
- Expert Scientific Support: Our experienced scientists provide consultation, data interpretation, and troubleshooting to support your goals from project start to finish.
- Fast Turnaround Time: We combine efficient workflows with in-house expertise to deliver high-quality results within competitive timelines—no delays, no compromises.
Case Study
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: β-Arrestin-independent endosomal cAMP signaling by a polypeptide hormone GPCR
Blythe and Von Zastrow, 2024. doi:10.1038/s41589-023-01412-4
This study challenges the prevailing view that β-arrestin is essential for endosomal GPCR–Gs–cAMP signaling. Using VIPR1, a secretin-family GPCR, researchers show that β-arrestin is not required for receptor internalization or endosomal signaling. Instead, β-arrestin modulates signaling dynamics by desensitizing plasma membrane responses, creating sequential cAMP peaks. The effect is linked to distinct β-arrestin–VIPR1 complexes at different cellular locations. Thus, endosomal GPCR signaling can occur independently of β-arrestin, which primarily shapes the timing and spatial aspects of the signal.
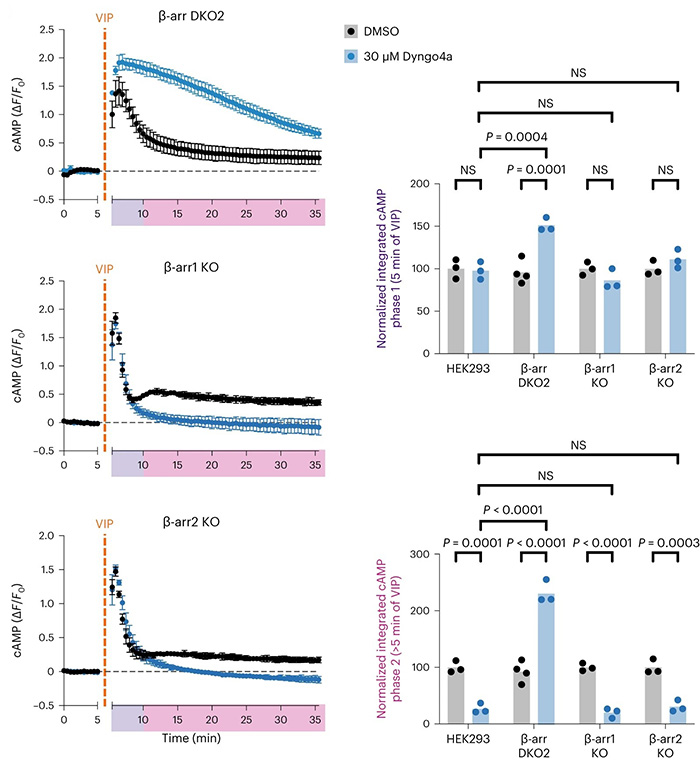
Figure 1. β-Arrestin is dispensable for VIPR1 endosomal cAMP signaling. Changes in cAMP in β-arr1/2 DKO line (β-arr DKO2) and β-arr1/2 single KO lines (β-arr1 KO and β-arr2 KO) upon treatment with 500 nM VIP added at 5 min. Cells were pretreated with 30 µM Dyngo4a (blue) or DMSO (black) for 10 min. Integrated cAMP of each phase (0–5 min or 5–30 min of VIP treatment) was calculated as the area under the curve and normalized to the average DMSO value for each cell line. (Blythe and Von Zastrow, 2024)
Case 2: Evaluation of 5-HT1A receptor agonism in psychotropic drugs across species using the [³⁵S]GTPγS binding assay
Odagaki et al., 2023. doi:10.1007/s43440-023-00448-6
This study assessed the 5-HT1A receptor agonist activity of several psychotropic drugs using [³⁵S]GTPγS binding assays in rat and human brain membranes. Brexpiprazole and aripiprazole showed agonist activity in both species, with aripiprazole demonstrating higher potency in rats, suggesting species differences. Vortioxetine showed efficacy at high concentrations but lacked clear potency. Lurasidone and asenapine exhibited minimal effects. Findings support brexpiprazole and vortioxetine as part of a pharmacological group defined by 5-HT1A agonism.
![Evaluation of 5-HT1A receptor agonism in psychotropic drugs acrossspecies using the [35S]GTPγS binding assay.](/images/gpcr-screening-assays-section-4-2.jpg)
Figure 2. Effects of 5-HT and several psychotropic drugs on specific [³⁵S]GTPγS binding in rat brain membranes prepared from hip pocampus (A), cerebral cortex (B), and striatum (C). Specific [³⁵S]GTPγS binding was measured in the presence of various concentrations of 5-HT (●), buspirone (▲), aripiprazole (○), brexpiprazole (△), lurasidone (▽), vortioxetine (□), and asenapine (◇). (Odagaki et al., 2023)
Customer Testimonials
-
"We partnered with this team for β-arrestin recruitment assays targeting Class B GPCRs. The data quality exceeded expectations, with excellent reproducibility across replicates. Creative BioMart’s team helped us identify a previously unrecognized biased agonist, which has since moved into our lead optimization pipeline. The support throughout was outstanding—they were responsive, technically knowledgeable, and flexible in accommodating our specific assay conditions."
— R&D Director | Mid-Sized Pharmaceutical Company
-
"Our project involved characterizing dimerization of dopamine receptor subtypes (D2/D3) using BRET-based assays. The results were robust and highly detailed, allowing us to explore functional consequences of receptor-receptor interactions that we couldn’t address in-house. Creative BioMart’s scientific input, especially during experimental design, was instrumental."
— Principal Investigator | Academic Neuroscience Lab
-
"We required G-protein dependent and independent assays to evaluate biased signaling of novel ligands targeting a GPCR involved in neuroinflammation. The Creative BioMart platform's ability to provide both cAMP and β-arrestin data under matched conditions was extremely helpful in interpreting the mechanism of action. The turnaround time was fast, the data analysis was comprehensive, and we appreciated the willingness to customize protocols for our unique scaffold series."
— Head of Discovery Biology | Biotech Startup (Neurology Focus)
-
"In a grant-funded project, we needed support with IP-One assays to quantify Gq-coupled receptor activity in primary human cells. Creative BioMart delivered not just technically sound data, but also helped troubleshoot aspects of receptor desensitization we hadn’t accounted for. Their willingness to engage in scientific discussion was refreshing."
— Group Leader of Translational Pharmacology Unit | Government Research Institute
FAQs
-
Q: What makes your GPCR Screening Assays stand out from others?
A: Our assays offer complete GPCR coverage—binding, signaling, dimerization—with high sensitivity, reproducibility, and flexibility, enabling detailed pharmacological profiling across multiple pathways. -
Q: How do your receptor binding assays ensure accuracy and consistency?
A: We apply validated protocols, controls, and quality checks using radioligand or non-radioligand formats to ensure consistent, reliable binding data across all experiments. -
Q: Can I detect biased signaling with your assays?
A: Yes, our assays assess both G-protein and β-arrestin pathways, allowing identification of functionally selective ligands and characterization of biased GPCR signaling. -
Q: Why should I care about GPCR dimerization, and do you test for that?
A: We offer BRET/FRET-based dimerization assays to study receptor-receptor interactions, providing insights into their role in signaling, ligand binding, and pharmacological responses. -
Q: How customizable are your assays for my specific target or pathway?
A: Assays are fully customizable—target selection, conditions, and detection methods—to meet project needs, including for orphan receptors or complex physiological models.
-
Q: What kind of data support and interpretation do you offer?
A: We deliver complete data packages, including quantitative results, curve fitting, and expert analysis, with ongoing support for interpretation and experimental troubleshooting.
Resources
Related Services
- Cytokine and Receptor Analysis
- cAMP Accumulation Assay
- Co-Immunoprecipitation (Co-IP) Service
- Stable Cell Line Services
- High-Throughput Screening
- Luciferase Reporter Assay
Related Products
References:
- Blythe EE, Von Zastrow M. β-Arrestin-independent endosomal cAMP signaling by a polypeptide hormone GPCR. Nat Chem Biol. 2024;20(3):323-332. doi:10.1038/s41589-023-01412-4
- Odagaki Y, Mikami M, Kinoshita M, et al. Agonistic properties of a series of psychotropic drugs at 5-HT1A receptors in rat and human brain membranes determined by [35S]GTPγS binding assay. Pharmacol Rep. 2023;75(2):266-275. doi:10.1007/s43440-023-00448-6
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

