IVD of Pet Allergy
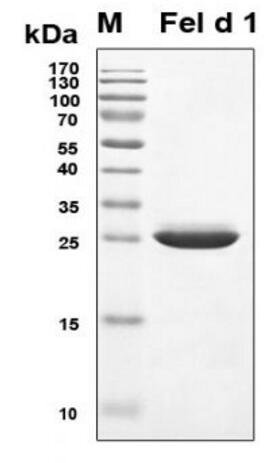
🧪 Feld1-4009C
Source: E.coli
Species: Cat
Tag: His&Trx
Conjugation:
Protein Length: 18-109 a.a.
Pet Allergy
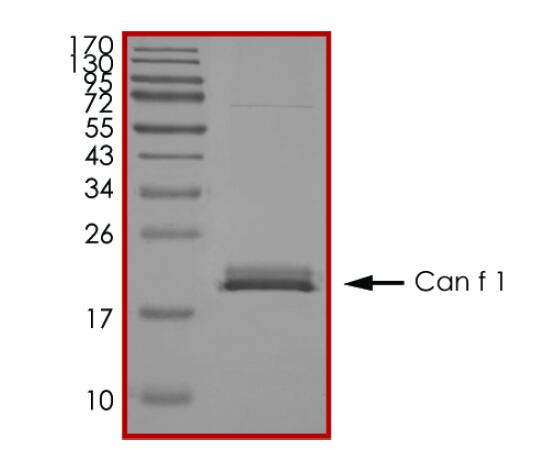
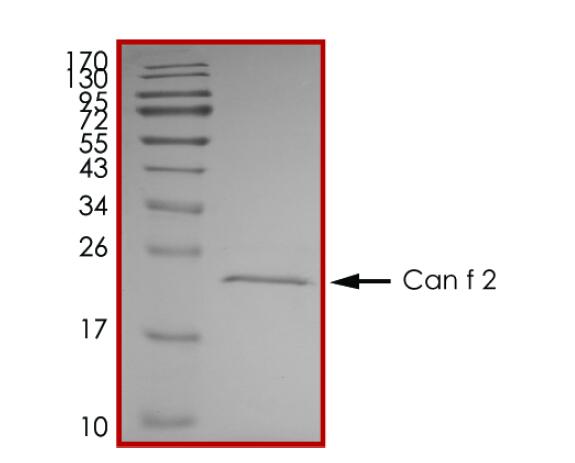
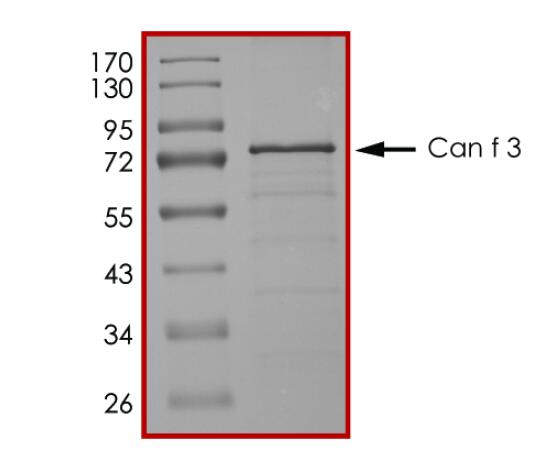
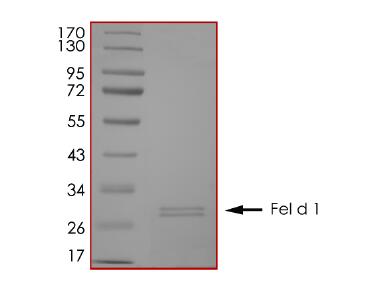
Pet allergy is an allergic reaction to proteins found in an animal's skin cells, saliva or urine. Pet allergies refer to symptoms such as nasal itching, runny nose, asthma, and rashes caused by allergies to pet hair and dander. Cats and dogs are the most common allergenic pets and have become the third largest category of inhaled allergens after dust mites and pollen allergens. There are currently 7 major allergenic proteins in dogs that have been identified, among which Can f 1 and Can f 5 are the main allergenic protein components. In addition, the main allergenic protein in cat hair is Fel d 1, while Equ c 1 is the secreted protein that induces IgE-mediated allergic reactions in most patients allergic to horses.

Main Steps of IVD for Pet Allergy
- Skin Test
It mainly includes intradermal tests and prick tests, which can detect the patient's sensitivity to allergens.
- Serum-Specific IgE Testing
This method can detect the content of pet allergen-specific IgE, such as specific IgE antibodies against cat hair, dog hair, or other pet hair, dander, and other components.
- Molecular Diagnostics
Modern molecular biology techniques are used to detect the gene expression of IgE antibodies that specifically bind to pet allergens in the human body to discover allergen-specific IgE and perform quantitative and qualitative detection.
Creative BioMart provides a diverse range of pet allergy-related recombinant allergen proteins used for IVD, including ELISA, lateral flow assay, western blot, and other immunoassays.
Highlights of Our Products
- Wide range of applications. Used for the development of various immunotherapies and vaccines as well as the production and preparation of various diagnostic reagents.
- High purity and specificity. Genetic engineering technology allows it to be produced without non-specific allergens.
- High sensitivity. The molecular structure and biological activity of recombinant allergens are similar to natural allergens and can effectively stimulate immune responses in allergic patients.
- Easy to scale up production.
- Higher safety and repeatability.
Our Outstanding Advantages
- The R&D team has rich experience and high-level technical capabilities and can provide you with continuously innovative products and services.
- A wide range of in-vitro diagnostic (IVD) products are available to meet diverse customer needs and support scientific research.
- Focus on service quality, provide customers with timely and accurate services, and ensure that customers can get the best scientific research experience.
Clinical Related Information
What are the symptoms for Pet Allergy?
- Sneezing
- Runny or stuffy nose
- Itchy, watery eyes
- Nasal congestion
- Coughing
- Wheezing and shortness of breath
- Rash or hives
- Itchy skin
How common are pet allergies?
Approximately 10-20% of the world's population is allergic to cats and dogs.
Cat allergies are about twice as common as dog allergies.
How to live with a pet if you're allergic?
It is possible to live with a pet if you are allergic, but it requires careful management and some lifestyle adjustments:
- Create Pet-Free Zones: Keep pets out of certain rooms, such as the bedroom.
- Use HEPA Filters: High-Efficiency Particulate Air filters can help reduce airborne pet allergens.
- Bathe and Groom Pets Regularly: Regular grooming and bathing can reduce the amount of pet dander.
- Clean Frequently: Frequent cleaning of your home, especially vacuuming carpets and furniture, can help reduce allergens.
Case Study
Case 1: Hemmer W, Sestak-Greinecker G, Braunsteiner T, Wantke F, Wöhrl S. Molecular sensitization patterns in animal allergy: Relationship with clinical relevance and pet ownership. Allergy. 2021 Dec;76(12):3687-3696. doi: 10.1111/all.14885. Epub 2021 May 31. PMID: 33914361.
To provide more information on prospective ligands, the researchers report crystal structures, NMR, molecular dynamics, and florescence studies of a dog lipocalin allergen Can f 1 and its closely related (and cross-reactive) cat allergen Fel d 7.
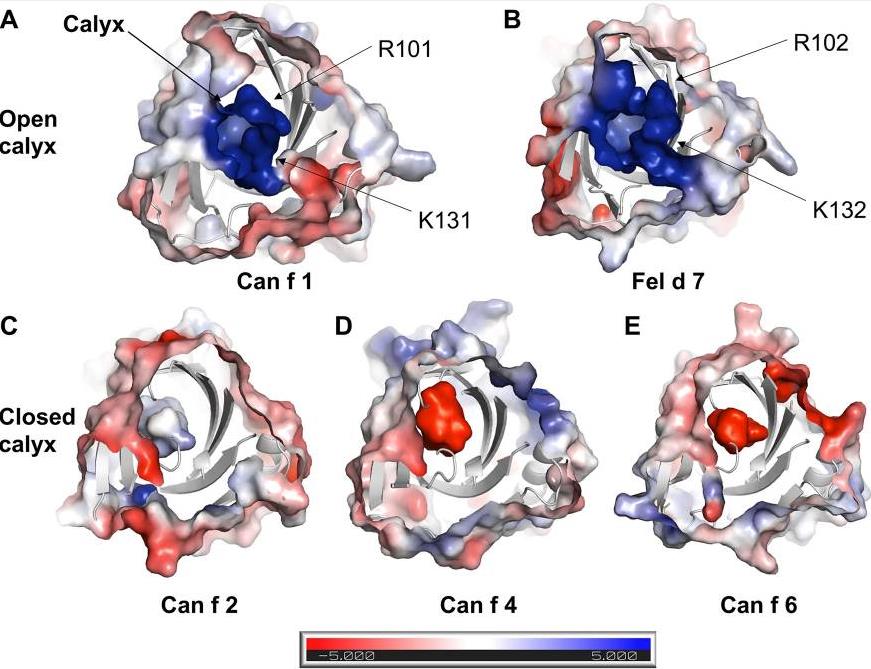 Fig2. Electrostatic surface of lipocalins. Electrostatic surface of (A) Can f 1, (B) Fel d 7, (C) Can f 2, (D) Can f 4, (E) Can f 6 are calculated using ABPS module in PyMol. The central calyx is shown with the arrow in A. Can f 1 and Fel d 7 show an open calyx while Can f 2, Can f 4, and Can f 6 show closed calyxes.
Fig2. Electrostatic surface of lipocalins. Electrostatic surface of (A) Can f 1, (B) Fel d 7, (C) Can f 2, (D) Can f 4, (E) Can f 6 are calculated using ABPS module in PyMol. The central calyx is shown with the arrow in A. Can f 1 and Fel d 7 show an open calyx while Can f 2, Can f 4, and Can f 6 show closed calyxes.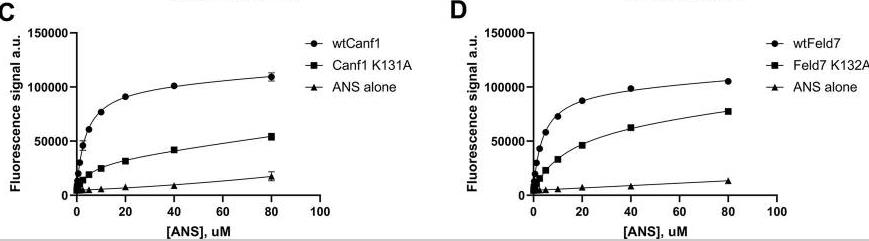 Fig3. ANS binding to Can f 1 and Fel d 7. The CD melting curve of wild-type and mutant proteins are measured at 210 nm in PBS buffer pH 7.4. The fluorescence increase upon ANS titration is shown for (C) WT Can f 1, Can f 1 (K131A), (D) WT Fel d 7, and Fel d 7 (K132A).
Fig3. ANS binding to Can f 1 and Fel d 7. The CD melting curve of wild-type and mutant proteins are measured at 210 nm in PBS buffer pH 7.4. The fluorescence increase upon ANS titration is shown for (C) WT Can f 1, Can f 1 (K131A), (D) WT Fel d 7, and Fel d 7 (K132A).Case 2: Matsuura R, Kawamura A, Ota R, Fukushima T, Fujimoto K, Kozaki M, Yamashiro M, Somei J, Matsumoto Y, Aida Y. TiO2-Photocatalyst-Induced Degradation of Dog and Cat Allergens under Wet and Dry Conditions Causes a Loss in Their Allergenicity. Toxics. 2023 Aug 21;11(8):718. doi: 10.3390/toxics11080718. PMID: 37624223; PMCID: PMC10458468.
Cleaning houses and washing pets are the main methods for reducing allergens in homes; however, it is difficult to eliminate them completely. Therefore, the authors aimed to investigate whether a TiO2 photocatalyst could degrade dog and cat allergens. The results indicated that the TiO2 photocatalyst degraded dog and cat allergens, causing a loss in their allergenicity. Their results suggest that TiO2 photocatalysis can be useful for removing indoor pet allergens and improving the partnership between humans and pets.
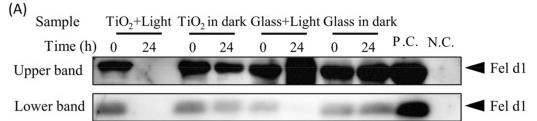 Fig4. Degradation of dog allergens in wet conditions. (A): To confirm whether the TiO2 photocatalyst degraded dog antigens in wet conditions, 100 μL of crude antigen was extracted from the dog hair coat, and the epithelium (contained 10 μg/mL of Can f1) was treated with TiO2 photocatalyst for 0 or 24 h in the "TiO2 + Light" group. As a control, the crude dog antigen was incubated with either the TiO2-coated glass sheet without LED light ("TiO2 in dark" group) or both the glass and LED light ("Glass + Light" group), or the glass without LED light ("Glass in the dark" group). Further, 150 ng of crude Can f1 antigen and PBS were used as positive and negative controls (P.C. and N.C.), respectively. Can f1 was detected by Western blot using anti-Can f1 monoclonal antibody (10D4). The positions of Can f1 are indicated.
Fig4. Degradation of dog allergens in wet conditions. (A): To confirm whether the TiO2 photocatalyst degraded dog antigens in wet conditions, 100 μL of crude antigen was extracted from the dog hair coat, and the epithelium (contained 10 μg/mL of Can f1) was treated with TiO2 photocatalyst for 0 or 24 h in the "TiO2 + Light" group. As a control, the crude dog antigen was incubated with either the TiO2-coated glass sheet without LED light ("TiO2 in dark" group) or both the glass and LED light ("Glass + Light" group), or the glass without LED light ("Glass in the dark" group). Further, 150 ng of crude Can f1 antigen and PBS were used as positive and negative controls (P.C. and N.C.), respectively. Can f1 was detected by Western blot using anti-Can f1 monoclonal antibody (10D4). The positions of Can f1 are indicated.