Principle and Protocol of Lowry Method
The Folin phenol reagent method consists of two steps. The first step is that under alkaline conditions, the protein reacts with copper to form a protein copper complex. The second step is that this complex will reduce Folin reagent to produce dark blue, and the color depth is proportional to the protein content. The quantitative range is 5-100 μg/mL protein. The color reaction of Folin reagent is caused by tyrosine, tryptophan and cysteine, so if the sample contains phenols, citric acid and mercapto compounds, they will interfere. In addition, the color intensity of different proteins is slightly different due to the different contents of tyrosine and tryptophan. The advantage of this method is that it is more sensitive than the biuret method, but the disadvantage is that the determination takes a long time and the operation time should be controlled accurately. The standard curve is not a strict straight-line form, and its specificity is poor, and there are many interfering substances.
The color development principle of Folin phenol reagent method is the same as that of biuret method, except that the second reagent, Folin phenol reagent, is added to increase the color development amount, thus improving the sensitivity of protein detection. The peptide bond of protein chelates with Cu2+ in alkaline solution to form protein copper complex. Phosphomolybdate phosphotungstate in Folin phenol reagent is reduced by tyrosine and phenylalanine residues in protein to produce dark blue substance. Under certain conditions, the color depth is proportional to the amount of protein. Therefore, the linear relationship between the blue depth and the protein concentration can be used as the standard curve and the protein concentration in the sample can be determined.
1. Main Instruments and Equipment
Micropipette, ultraviolet visible spectrophotometer, quartz cuvette, balance, vortex oscillator.
2. Experimental Materials
Protein sample to be tested.
3. Main reagents
Sample solution*1
Protein standard solution (1mg/mL): accurately weigh 1 g of bovine serum albumin and prepare 250 mg/mL with 4 mL of sample solution.
Reagent A:
(A) 10 g Na2CO3, 2 g NaOH and 0.25 g potassium sodium tartrate (KNaC4H4O6 · 4H2O) were dissolved in 500 mL distilled water.
(B) 0.5g copper sulfate (CuSO4 · 5H2O) is dissolved in 100mL distilled water. Before each use, reagent (A) and (B) are mixed at a ratio of 50:1, which is reagent A. The reagent should be used within 24h, expired*2.
Reagent B:
In a 2L grinded reflux bottle, add 100g of sodium tungstate (Na2WO4 · 2H2O), 5g of sodium molybdate (Na2MoO4 · 2H2O) and 700mL of distilled water, then add 50mL of 85% phosphoric acid and 100mL of concentrated hydrochloric acid, fully mix them, connect the reflux pipe, and reflux with low fire for 10h. At the end of reflux, add 150g of lithium sulfate (Li2SO4), 50mL of distilled water and several drops of liquid bromine, and continue boiling at the opening for 15min to remove excess bromine. After cooling, the solution turns yellow (if it still turns green, repeat the steps of adding liquid bromine). Dilute to 1L and filter, and store the filtrate in a brown reagent bottle. When using, titrate with standard NaOH, use phenolphthalein as the indicator, dilute it appropriately, and add about twice as much water to make the final acid concentration about 1mol/L (preparation is difficult and complicated, and it can now be purchased separately as a reagent).
1. Standard Curve Method
(1) Take 7 tubes and add reagents according to Table 2-1-4
Table 2-1-4 Preparation of Protein Standard Curve*3
| Reagent | Test tube number | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Test tube | ||
| Standard solution /mL | - | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | - | |
| Sample solution /mL | 1.0 | 0.8 | 0.6 | 0.4 | 0.2 | - | - | |
| Sample to be tested /mL | 1.0 | |||||||
| Reagent A /mL | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | |
| Quickly mix on the vortex oscillator and place it at room temperature (20-25 °C) for 10 minutes | ||||||||
| Reagent B /mL | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Protein mass concentration/ (mg/mL) | - | 50 | 100 | 150 | 200 | 250 | ||
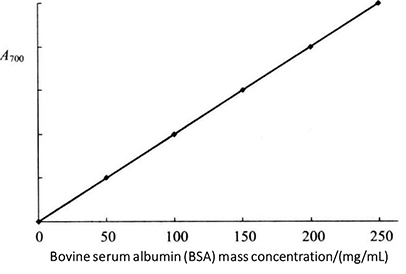 Figure 2-1-4 Standard Curve of Folin-phenol Reagent Method
Figure 2-1-4 Standard Curve of Folin-phenol Reagent Method
(2) Place at room temperature for 30min, take the first tube without protein solution as blank control, and measure the absorbance value of solution in each tube at 700nm. The standard curve is drawn with the mass concentration of protein (mg/mL) as the abscissa and the absorbance as the ordinate (Figure 2-1-4).
2. Determination of Protein Content in Samples
Dissolve 1ml of protein sample to be tested (20~250mg/mL) according to the above method. Generally, the determination of samples can also be carried out together with the determination of standard curves*4. After each test tube measured by the standard curve, add another measuring tube. According to the absorbance value of the measured sample, find out the corresponding protein mass on the standard curve, and then calculate the protein mass concentration of the sample solution.
1. During the determination, special care should be taken when adding Folin phenol reagent, because the reagent is stable only under the acidic pH condition, but the reduction reaction only occurs when pH=10, so when Folin phenol reagent is added to the alkaline copper protein solution, it must be immediately mixed, so that the reduction reaction can occur before the phosphomolybdic acid phosphotungstic acid reagent is destroyed.
2. As the color development of Lowry reaction deepens with time, the time of each operation must be precisely controlled, that is, after adding 5ml of reagent A to the first tube, start timing, after 1min, add 5ml of reagent A to the second tube, after 2 min, add the third tube, and so on, not more than 10min.
3. Because various proteins contain different amounts of tyrosine and phenylalanine, the color depth often varies with different proteins. Therefore, this method is usually only applicable to determining the relative concentration of protein (relative to standard protein).
* 1 This can be distilled water, protein buffer solution or salt solution. Depending on the sample dissolution solution.
* 2 Dispense the amount to be used on the day as required.
* 3 Standard solution preparation.

*4 At this point, it is important to ensure that the concentration of the standard is accurate.

