IVD of Dermatophagoides farina Allergy
🧪 DERF1-907D
Source: HEK293
Species: Dermatophagoides farinae
Tag: His
Conjugation:
Protein Length:

🧪 DERF2-908D
Source: E.coli
Species: Dermatophagoides farinae
Tag: His
Conjugation:
Protein Length:

🧪 Der f 1-08D
Source: E.coli
Species: Dermatophagoides farinae
Tag: His
Conjugation:
Protein Length:
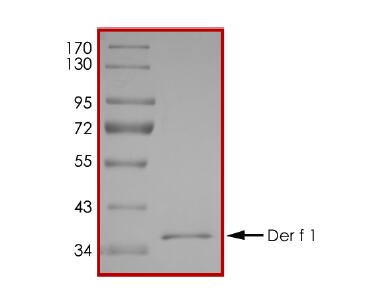
🧪 Der f 2-09D
Source: E.coli
Species: Dermatophagoides farinae
Tag: His
Conjugation:
Protein Length:
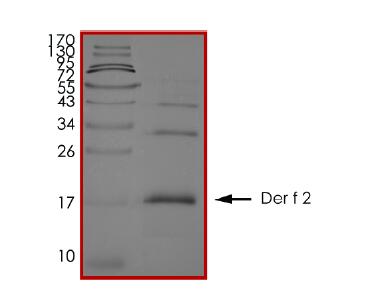
🧪 DERF2-3809D
Source: E.coli
Species: Dermatophagoides farinae
Tag: His&SUMO
Conjugation:
Protein Length: 18-146aa

🧪 Der f 1 allergen-1558D
Source: Insect Cells
Species: Dermatophagoides farinae
Tag: His
Conjugation:
Protein Length: Met1-Met321

Dermatophagoides farinae
Dermatophagoides farinae (Der f) is the main source of inhaled allergens and is highly related to the occurrence of allergic diseases. Der f allergens refer to the protein components of Der f, including their corpses, secretions, excretions, and eggs. When these substances are spread in the air and inhaled by the human body, they can cause allergic rhinitis, allergic asthma, urticaria, dermatitis, and a series of other symptoms. Currently, immunological detection methods such as ELISA are usually used to detect specific IgE antibodies against Der f 2 allergens in patients' serum to diagnose dust mite allergy in vitro.
Main Steps of IVD for Dermatophagoides farinae Allergy
- Immunological Test
Methods mainly include ELISA, skin prick test, and western blot, among which ELISA is one of the most commonly used methods. During the IVD process, the allergen protein of Der f 2 is combined with the specific IgE antibody in the patient's serum for immunological testing to determine whether the patient has an immune response to the allergens of Der f, thereby helping to diagnose allergy. - Molecular Biology Test
The main methods are gene cloning, gene expression, and protein analysis, which can detect the genes and proteins of Der f to further confirm the allergens of Der f.
Causes of Dermatophagoides farina Allergy
Exposure to Dust Mites:
Dust mites thrive in warm, humid environments and are commonly found in bedding, upholstered furniture, carpets, and curtains.
Allergen Proteins:
Proteins found in the mite's body, feces, and secretions can trigger allergic reactions. These allergens are easily inhaled or come into contact with the skin.
Environmental Factors:
Poor ventilation, high humidity, and accumulation of dust can increase the population of dust mites indoors, thus increasing the risk of exposure.
Genetic Predisposition:
Some individuals may be more genetically susceptible to developing allergies, including those caused by dust mites.
Symptoms of Dermatophagoides farina Allergy
When someone allergic to Dermatophagoides farina is exposed to the mites or their waste products, they may experience a variety of symptoms, including:
Respiratory Symptoms:
- Sneezing
- Runny or stuffy nose (allergic rhinitis)
- Itchy or watery eyes
- Coughing
- Wheezing
- Shortness of breath
- Asthma exacerbation
Skin Symptoms:
- Itchy skin
- Eczema (atopic dermatitis)
- Red, irritated skin
Other Symptoms:
Fatigue due to poor sleep quality from nasal congestion
Sinus pain or pressure
Creative BioMart provides high-quality recombinant allergen Der f used for IVD, including ELISA, lateral flow assay, western blot, and other immunoassays.
Highlights of Our Products
- High purity, no non-specific allergen contamination.
- High specificity. The molecular structure and biological activity of recombinant allergens are similar to natural allergens and can effectively stimulate immune responses in allergic patients.
- Easy to scale up production. A large number of specific proteins can be obtained through process amplification, without being limited by the content of natural extracts and materials.
- Higher safety and repeatability.
Our Outstanding Advantages
- Strong technical team, advanced scientific research equipment, and technology that can provide high-quality services.
- A rich variety of IVD products to meet the different needs of customers and provide customers with comprehensive scientific research support.
- Pay attention to service quality, provide customers with timely and accurate IVD-related services, and ensure that customers can get the best scientific research experience.
Case Study
Case 1: Yang T, Xu Z, Yu J, Liu J, Wang W, Hong S. Exosomes Derived from Dermatophagoides farinae Induce Allergic Airway Inflammation. Microbiol Spectr. 2023 Aug 17;11(4):e0505422. doi: 10.1128/spectrum.05054-22. Epub 2023 Jun 14. PMID: 37314339; PMCID: PMC10434197.
This study, for the first time, extracted exosomes from D. farinae, and sequenced their protein cargo and microRNAs using shotgun liquid chromatography-tandem mass spectrometry and small RNA sequencing. D. farinae-derived exosomes trigger allergen-specific immune responses and present satisfactory immunogenicity, as revealed by immunoblotting, Western blotting, and enzyme-linked immunosorbent assay and may induce allergic airway inflammation via bronchial epithelial cells and alveolar macrophages. Their data provide insights into the mechanisms of allergic airway inflammation caused with D. farinae-derived exosomes and the treatment of house dust mite-induced allergic airway inflammation.
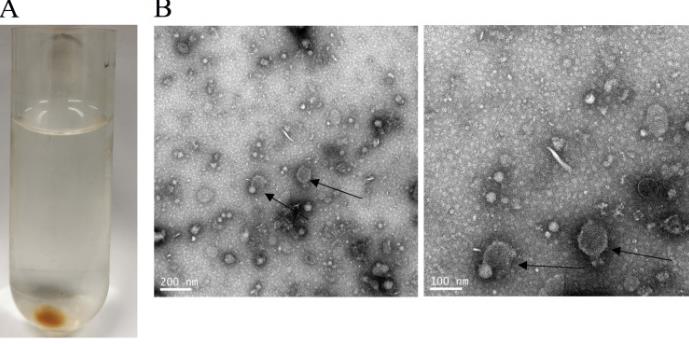 Fig2. Characterization of D. farinae exosomes. (A) Light-yellow gel-like pellet is acquired after ultracentrifugation. Transmission electron microscopy (B).
Fig2. Characterization of D. farinae exosomes. (A) Light-yellow gel-like pellet is acquired after ultracentrifugation. Transmission electron microscopy (B).Case 2: Kim JY, Yi MH, Kim M, Choi JH, Lee S, Yong TS. Production of Dermatophagoides farinae Having Low Bacterial Content Using Ampicillin. J Immunol Res. 2023 May 18;2023:9024595. doi: 10.1155/2023/9024595. PMID: 37252681; PMCID: PMC10212681.
The researchers showed that bacterial content in D. farinae was reduced by ampicillin treatment, which was sufficient to induce allergic sensitization and an immune response. This method will be used to develop more controlled allergy immunotherapeutic agents.
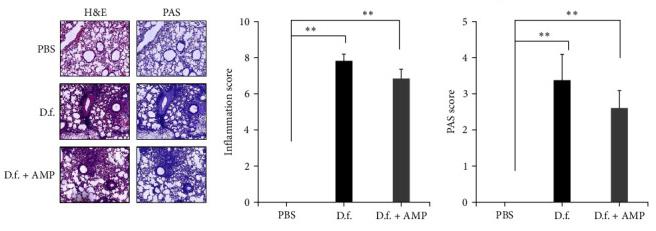 Fig3. Induction of airway hyperreactivity with ampicillin treated-Dermatophagoides farinae (D.f. + AMP) and untreated D. farinae (D.f.) in vivo. Hematoxylin and eosin (H&E), periodic acid-schiff (PAS), and Masson's trichrome staining of the lung sections.
Fig3. Induction of airway hyperreactivity with ampicillin treated-Dermatophagoides farinae (D.f. + AMP) and untreated D. farinae (D.f.) in vivo. Hematoxylin and eosin (H&E), periodic acid-schiff (PAS), and Masson's trichrome staining of the lung sections.Case 3: Jin M, Bang JS, Ha DL, Kim JY, Park KD, Lee WJ, Lee SJ, Choi JK, Choi YA, Jang YH, Kim SH. Dermatophagoides farinae Extract Induces Interleukin 33-Mediated Atopic Skin Inflammation via Activation of RIP1. Int J Mol Sci. 2023 Mar 9;24(6):5228. doi: 10.3390/ijms24065228. PMID: 36982304; PMCID: PMC10049056.
The authors examined the role of RIP1 in Dermatophagoides farinae extract (DFE)-induced atopic dermatitis (AD)-like skin inflammation. RIP1 phosphorylation was increased in HKCs treated with DFE. Nectostatin-1, a selective and potent allosteric inhibitor of RIP1, inhibited AD-like skin inflammation and the expression of histamine, total IgE, DFE-specific IgE, IL-4, IL-5, and IL-13 in an AD-like mouse model.
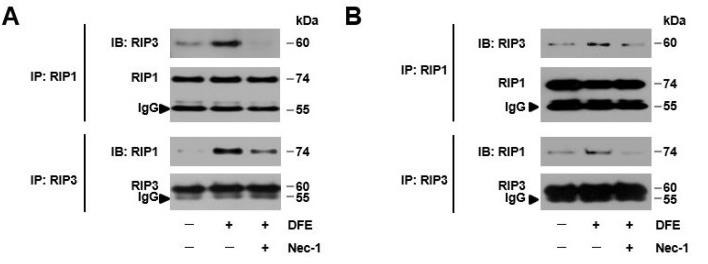 Fig4. Dermatophagoides farinae extract (DFE) activates RIP1 expression in vitro, in a DFE-induced mouse model with AD-like skin lesions, and in the lesional skin of atopic dermatitis patients with house dust mite (HDM) sensitization. (A,B) HaCaT cells or HKCs were stimulated with DFE (100 μg/mL) for immunoprecipitation (IP) with anti-phospho-RIP1, anti-RIP1, or anti-RIP3 antibodies. Phospho-RIP1, RIP1, and RIP3 protein levels from whole-cell lysates were determined using immunoblotting.
Fig4. Dermatophagoides farinae extract (DFE) activates RIP1 expression in vitro, in a DFE-induced mouse model with AD-like skin lesions, and in the lesional skin of atopic dermatitis patients with house dust mite (HDM) sensitization. (A,B) HaCaT cells or HKCs were stimulated with DFE (100 μg/mL) for immunoprecipitation (IP) with anti-phospho-RIP1, anti-RIP1, or anti-RIP3 antibodies. Phospho-RIP1, RIP1, and RIP3 protein levels from whole-cell lysates were determined using immunoblotting.

