IVD of Caw Milk Allergy
🧪 IgG-129B
Source: Bovine Milk
Species: Bovine
Tag: Non
Conjugation:
Protein Length: Full length native Bovine milk Immunoglobulin G

🧪 MBLG-167B
Source: Bovine Milk
Species: Bovine
Tag: Non
Conjugation:
Protein Length: Full length native Bovine milk β-Lactoglobulin
Background
Milk is a nutritious food, but some people experience allergic reactions after drinking milk. 80% of milk protein is made up of casein, while the remaining 20% comprises whey protein, which is considered the main trigger for milk allergy. Clinical allergen research has found that β-lactoglobulin and a-casein in milk are the most common allergens, which can cause symptoms such as rash, vomiting, diarrhea, and wheezing. Infants and young children have the highest rate of allergy to milk protein due to their immature intestinal mucosal barrier and immune system. Currently, blood tests for specific IgE can be used to assist in the diagnosis of IgE-related milk protein allergy.

Symptoms of Milk Allergy
- Skin reactions: Hives, rash, itching, and eczema.
- Gastrointestinal symptoms: Abdominal pain, colic, diarrhea, nausea, and vomiting.
- Respiratory symptoms: Wheezing, coughing, runny nose, and shortness of breath.
- Anaphylaxis: A severe, potentially life-threatening allergic reaction that requires immediate medical attention.
What Does Milk Allergy Look Like in Adults and Infants?
- In Infants: Symptoms often include skin reactions such as eczema, gastrointestinal symptoms like colic, diarrhea, blood in stools, and vomiting. Respiratory symptoms may also be present.
- In Adults: Symptoms may also include gastrointestinal distress, skin reactions, asthma-like symptoms, and in severe cases, anaphylaxis. Adults might experience more pronounced gastrointestinal symptoms compared to infants.
Main Steps of IVD for Caw Milk Allergy
- IgE Antibody Test
Through the fluorescent enzyme immunoassay, the patient's serum sample is taken and the total amount of specific IgE in the patient's sample is checked to determine the allergy.
- Skin Prick Test
Drop a small amount of highly purified allergen solution on the patient's forearm skin, then use a puncture needle to gently penetrate the skin surface, and finally wait 15 to 20 minutes to observe the skin condition. If there is an allergic reaction to the allergen, symptoms such as local wheals, erythema, and itching will occur.
- Oral Food Challenge (OFC)
Implement gradually from low concentration to high concentration.
- Tryptase Test
Venous blood collection or 24-hour urine collection can be used to confirm whether the patient is allergic to milk.
Creative BioMart provides high-quality recombinant caw milk allergen protein used for IVD, including ELISA, lateral flow assay, western blot, and other immunoassays.
Highlights of Our Products
- High sensitivity and specificity. The protein can accurately identify and detect allergens.
- Easy to scale up production.
- It is widely used and suitable for downstream immunological experiments.
- Higher safety and repeatability.
- Excellent success rate and rapid development.
Our Outstanding Advantages
- Multi-range IVD products meet the different needs of customers and provide customers with comprehensive scientific research support.
- Provide customers with timely and accurate IVD-related services, and ensure that customers can get the best scientific research experience.
- We offer exceptional service with a robust technical team, state-of-the-art scientific research equipment, and technology.
- Deliver quickly and complete target requirements within a short period.
Case Study
Case 1: Darma A, Sumitro KR, Jo J, Sitorus N. Lactose Intolerance versus Cow's Milk Allergy in Infants: A Clinical Dilemma. Nutrients. 2024 Jan 31;16(3):414. doi: 10.3390/nu16030414. PMID: 38337698; PMCID: PMC10856892.
Lactose intolerance (LI) and cow's milk allergy (CMA) are the most common adverse reactions to cow's milk. This review is therefore written to assist clinicians to better understand the pathophysiologies of LI and CMA as well as to recognize the similarities and differences between clinical manifestations of LI and CMA.
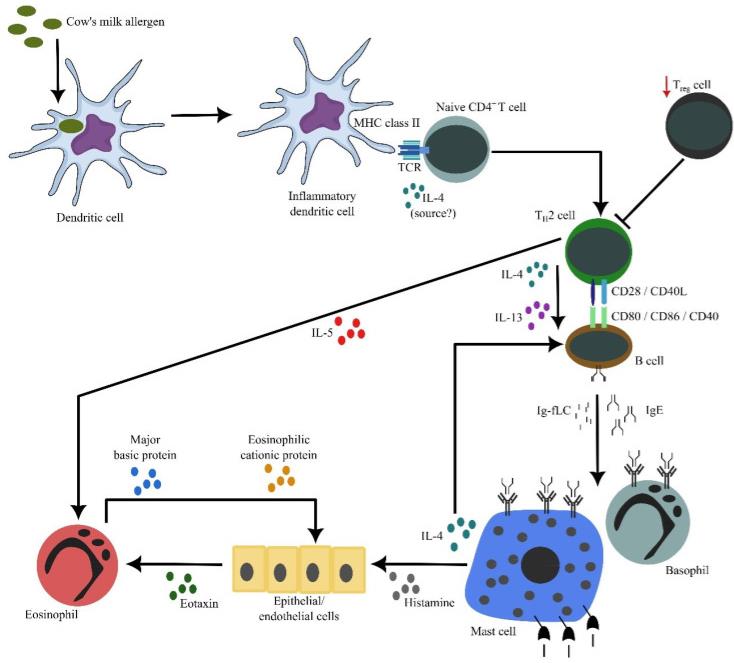 Fig2. The cascade of inflammation in cow's milk allergy. Allergens' exposure to inflammatory dendritic cells allow these cells to process and present allergen-derived peptides to naïve CD4+ T cells. In the presence of IL-4 (from an unknown source), naïve CD4+ T cells differentiate into proallergic TH2 cells. Concurrently, it appears that there is an impairment of TReg cell frequency and/or activity, resulting in a lack of suppression of TH2 cell activity. Subsequently, TH2 cells will drive B cells, via cell contact as well as secreted IL-4 and IL-13, to undergo immunoglobulin class switch recombination, in which they eventually produce IgE. Along with antibody production, B cells also secrete significant amounts of κ and λ Ig-free light chains (Ig-fLCs). IgE and Ig-fLCs will then bind to mast cells and basophils, causing sensitization. Following subsequent exposure to allergens, cross-linking of surface-bound antibodies occurs, causing mast cells and basophils to degranulate and release their biologically active substances, including histamine, IL-4 and IL-5. Secreted IL-4 amplifies the differentiation between TH2 and IgE-producing B cells, while secreted IL-5 by TH2 cells causes accumulation and activation of eosinophils in the affected tissues. Similarly, histamine causes epithelial or endothelial cells to release eotaxin that attracts eosinophils into the tissues. Activated eosinophils release active substances, including major basic and eosinophilic cationic proteins that are toxic to the surrounding cells, contributing to further inflammation.
Fig2. The cascade of inflammation in cow's milk allergy. Allergens' exposure to inflammatory dendritic cells allow these cells to process and present allergen-derived peptides to naïve CD4+ T cells. In the presence of IL-4 (from an unknown source), naïve CD4+ T cells differentiate into proallergic TH2 cells. Concurrently, it appears that there is an impairment of TReg cell frequency and/or activity, resulting in a lack of suppression of TH2 cell activity. Subsequently, TH2 cells will drive B cells, via cell contact as well as secreted IL-4 and IL-13, to undergo immunoglobulin class switch recombination, in which they eventually produce IgE. Along with antibody production, B cells also secrete significant amounts of κ and λ Ig-free light chains (Ig-fLCs). IgE and Ig-fLCs will then bind to mast cells and basophils, causing sensitization. Following subsequent exposure to allergens, cross-linking of surface-bound antibodies occurs, causing mast cells and basophils to degranulate and release their biologically active substances, including histamine, IL-4 and IL-5. Secreted IL-4 amplifies the differentiation between TH2 and IgE-producing B cells, while secreted IL-5 by TH2 cells causes accumulation and activation of eosinophils in the affected tissues. Similarly, histamine causes epithelial or endothelial cells to release eotaxin that attracts eosinophils into the tissues. Activated eosinophils release active substances, including major basic and eosinophilic cationic proteins that are toxic to the surrounding cells, contributing to further inflammation.Case 2: Roth-Walter F, Afify SM, Pacios LF, et al. Cow's milk protein β-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells. J Allergy Clin Immunol. 2021 Jan;147(1):321-334.e4. doi: 10.1016/j.jaci.2020.05.023. Epub 2020 May 30. PMID: 32485264.
Beta-lactoglobulin (BLG) is a bovine lipocalin in milk with an innate defense function. The circumstances under which BLG is associated with tolerance of or allergy to milk are not understood. The authors' aims were to assess the capacity of ligand-free apoBLG versus loaded BLG (holoBLG) to protect mice against allergy by using an iron-quercetin complex as an exemplary ligand and to study the molecular mechanisms of this protection.
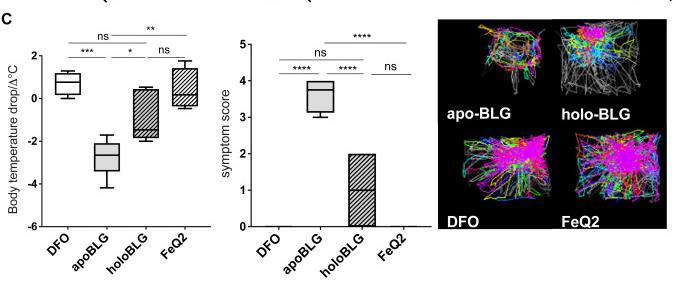 Fig3. Body temperature drop, symptom score, and representative images of body temperature (blue to red indicates low to high temperature) and movements (lines) recorded by the imaging cage after systemic challenge with BLG in the different treated groups. Representative data from 3 independent experiments are shown.
Fig3. Body temperature drop, symptom score, and representative images of body temperature (blue to red indicates low to high temperature) and movements (lines) recorded by the imaging cage after systemic challenge with BLG in the different treated groups. Representative data from 3 independent experiments are shown.








