Protein Ubiquitination Service
Creative BioMart offers comprehensive Protein Ubiquitination Services, designed to support both fundamental research and drug discovery programs. Our platform enables precise profiling of ubiquitin incorporation into target proteins using validated E3 ligase cascades and functional high-content microarrays. We provide high-quality, quantitative assays to detect ubiquitination events and screen for modulators, including inhibitors and activators. By leveraging our expertise in ubiquitin biology, we help researchers identify key E3 ligases, elucidate substrate specificity, and accelerate mechanistic studies. Our service is suitable for diverse experimental needs, from small-scale research projects to high-throughput compound screening campaigns.
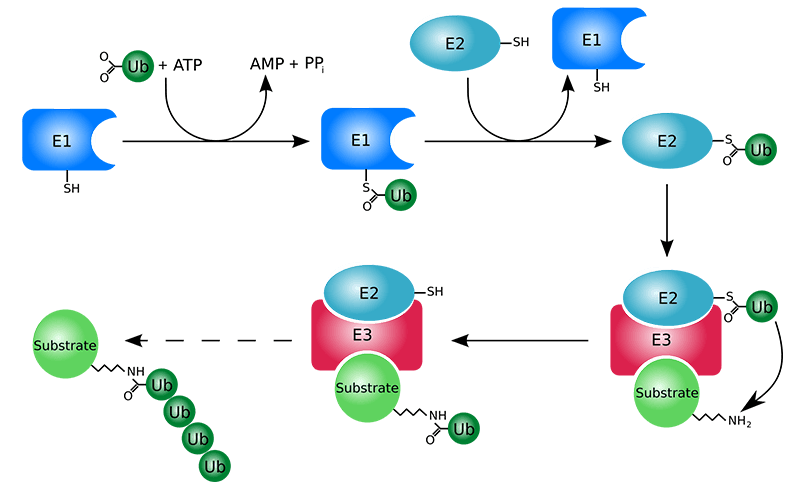
Understanding Protein Ubiquitination
Protein ubiquitination is a post-translational modification where ubiquitin molecules are covalently attached to lysine residues on target proteins. This process, orchestrated by E1 activating enzymes, E2 conjugating enzymes, and E3 ligases, plays critical roles in protein degradation, signal transduction, DNA repair, and cellular homeostasis. Dysregulation of ubiquitination is implicated in cancer, neurodegeneration, inflammation, and other diseases, making it a key area of study for both basic research and therapeutic development. Accurate profiling of ubiquitination and identification of E3 ligases are essential for understanding protein regulation and discovering novel drug targets.
Comprehensive Protein Ubiquitination Services
Creative BioMart provides a broad spectrum of protein ubiquitination services:
-
Ubiquitin Screening Service
Quantitative profiling of substrates against validated E3 ligase cascades using electrochemiluminescence detection.
-
E3 Ligase Identification Service
High-content microarray-based identification of candidate E3 ligases capable of modifying your substrate.
-
Custom Assay Design
Optimization of assay conditions to accommodate unique substrates, protein tags, or compound libraries.
-
Modulator Screening
Evaluation of inhibitors, enhancers, and small molecules targeting the ubiquitination pathway.
Service Highlights
- Validated assays for an expanding panel of E3 ligase cascades.
- Biologically relevant substrates used in all assays.
- Quantitative detection allows profiling at single or multiple concentrations.
- High-content microarrays enable precise E3-substrate identification.
- Flexible formats suitable for small-scale or high-throughput studies.
- Expertise from a team with years of experience in ubiquitin biology.
Service Workflow

Why Partner with Creative BioMart
- Comprehensive Expertise: Decades of experience in protein ubiquitination assays and pathway analysis.
- High-Quality Assays: Validated, reproducible assays for a growing panel of E3 ligases and substrates.
- Flexible Services: Support for small-scale research, high-throughput screening, and custom assay formats.
- Quantitative & Reliable: Sensitive electrochemiluminescence and microarray-based detection ensure accurate results.
- End-to-End Support: From experimental design to data analysis and reporting.
- Global Reach: Trusted by academic and pharmaceutical researchers worldwide for ubiquitination studies.
Case Studies and Real-World Applications
Case 1: Activity-based E3 ligase profiling uncovers an E3 ligase with esterification activity
Pao et al., 2018. doi:10.1038/s41586-018-0026-1
RING E3s transfer ubiquitin directly from E2 to substrates, HECT E3s form a covalent E3–Ub intermediate, and RBR E3s combine RING and HECT features. Recent activity-based profiling of HECT- and RBR-like ligases identified the neuron-associated E3 ligase MYCBP2 (PHR1) as a unique RING-linked ligase with esterification activity selectively targeting threonine. This RING-Cys-relay (RCR) mechanism, supported by structural studies, reveals previously unappreciated E3 mechanistic diversity and highlights non-lysine ubiquitination in higher eukaryotic regulation.
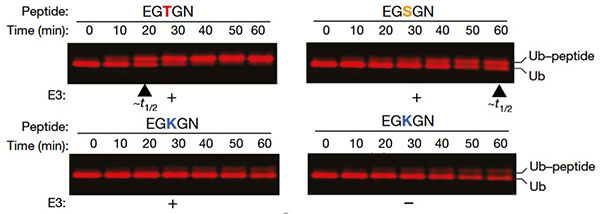
Figure 1. SDS–PAGE analysis of peptide modification with fluorescently labelled ubiquitin. Based on half-life approximation, MYCBP2(cat) has a threefold selectivity for threonine over serine in the depicted peptide context. (Pao et al., 2018)
Case 2: SOCS2-mediated radio sensitization in hepatocellular carcinoma
Chen et al., 2023. doi:10.1038/s41418-022-01051-7
Radio resistance limits the effectiveness of radiotherapy in hepatocellular carcinoma (HCC), and the regulatory mechanisms remain poorly understood. This study identified suppressor of cytokine signaling 2 (SOCS2) as a potential prognostic biomarker for HCC radiotherapy through RNA-seq and bioinformatics analyses. SOCS2 was shown to enhance radiosensitivity both in vitro and in vivo by promoting ferroptosis. Mechanistically, SOCS2 binds the N-terminal domain of SLC7A11 and, via its BOX region interacting with elongin B/C, facilitates K48-linked polyubiquitination and degradation of SLC7A11. This degradation increases ferroptosis, enhancing HCC radiosensitivity. High SOCS2 expression thus predicts radiosensitivity and may represent a therapeutic target to improve radiotherapy outcomes.
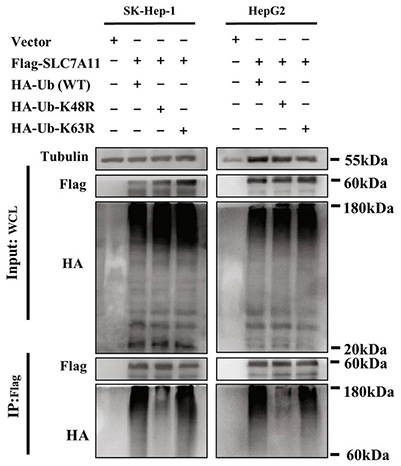
Figure 2. Immunoprecipitation and immunoblotting assay were selected to detect the type of polyubiquitination of Flag-SLC7A11 in SK-Hep-1 and HepG2 cells transfected with wild-type or K48/K63 mutant Ub after IR. (Chen et al., 2023)
What Our Customers Say
“Creative BioMart’s ubiquitination service was instrumental in our E3 ligase inhibitor screening project. Their team efficiently set up cascades with our target substrate and provided clear, quantitative electrochemiluminescence readouts. The data quality exceeded our expectations, allowing us to identify several promising lead compounds for further development.”
— Senior Scientist | Mid-Sized Biopharmaceutical Company
“We engaged Creative BioMart to identify potential E3 ligases for a novel oncology target. Their high-content microarray analysis quickly revealed several candidate ligases, saving us months of trial-and-error experiments. The team’s insights into substrate selection and assay design were invaluable.”
— R&D Director | Academic Research Institute
“Creative BioMart supported our project on ubiquitin pathway modulation for neurodegenerative disease. Their ubiquitination assays were reproducible across multiple batches, and the team provided thorough guidance on enzyme-substrate selection, streamlining our compound profiling workflow.”
— Principal Investigator | Pharmaceutical Company
“We relied on Creative BioMart to perform ubiquitination profiling for a series of novel protein targets. Their ability to run assays at multiple concentrations and provide biologically-relevant substrates allowed us to generate robust, high-confidence data that directly informed our drug discovery pipeline.”
— Associate Director, Protein Chemistry | Global Biotech Firm
FAQs About Protein Ubiquitination Services
-
Q: What types of ubiquitination assays do you provide?
A: We offer both ubiquitin screening assays and E3 ligase identification services. Our screening assays quantify ubiquitin incorporation into biologically relevant substrates using electrochemiluminescence, enabling identification of inhibitors and activators. The E3 ligase identification service uses high-content microarrays to discover ligases capable of ubiquitinating your protein of interest. -
Q: Can you work with custom substrates?
A: Yes. We can accommodate client-provided substrates or help select the most appropriate substrate for your experimental goals. Direct labeling of proteins for detection is also available to enhance assay sensitivity. -
Q: How scalable are your assays?
A: Our assays support single- or multiple-concentration testing, making them suitable for both small exploratory studies and large high-throughput compound screening campaigns. -
Q: Are your services suitable for complex or novel targets?
A: Absolutely. Our team has extensive experience with diverse E3 ligase cascades and can advise on optimal experimental design, ensuring reliable results even for novel or challenging proteins. -
Q: How is data delivered and analyzed?
A: Clients receive complete datasets including raw signals, normalized results, and clear summaries of E3-substrate interactions. Our experts provide guidance on interpreting results and next steps for downstream applications.
Resources
Related Services
Related Products
References:
- Chen Q, Zheng W, Guan J, et al. SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 2023;30(1):137-151. doi:10.1038/s41418-022-01051-7
- Pao KC, Wood NT, Knebel A, et al. Activity-based E3 ligase profiling uncovers an E3 ligase with esterification activity . Nature. 2018;556(7701):381-385. doi:10.1038/s41586-018-0026-1
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

