Endotoxin-free Protein Production
As demand grows for high-purity recombinant proteins for use in vaccines, therapeutics, diagnostics, and cell-based assays, eliminating endotoxin contamination has become critical. Lipopolysaccharides (LPS), the primary endotoxin component of E. coli , can cause severe issues in mammalian systems, from triggering immune responses to killing cultured cells. To meet the needs of researchers and product developers working in in vivo models, cell therapy, and drug discovery, Creative BioMart offers a robust Endotoxin-Free Protein Production Service . Using our proprietary endotoxin-free E. coli expression system , we deliver biologically active, high-yield proteins with ultra-low endotoxin levels—ready for your most sensitive applications.
Background: The Need for Endotoxin-Free Protein Expression
Recombinant proteins produced in Escherichia coli are extensively utilized in academic research, pharmaceutical development, and industrial biotechnology. However, a significant limitation of conventional E. coli expression systems is endotoxin contamination, primarily caused by lipopolysaccharides (LPS), which constitute a major structural component of the bacterial outer membrane. With approximately 2 million LPS molecules per cell—comprising roughly 30% of the outer membrane mass—even minimal contamination can severely affect downstream applications.
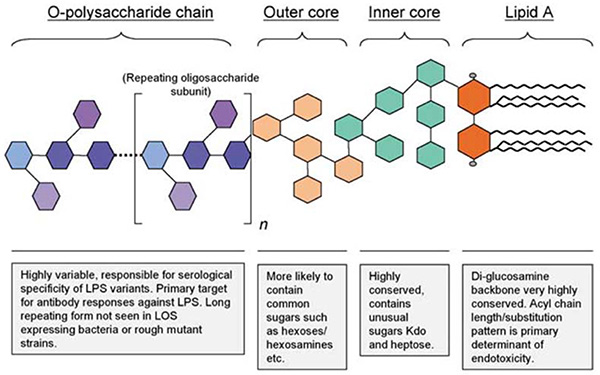
Figure 1. The general structure of Gram-negative bacterial lipopolysaccharide (endotoxin). (Eckel and Ametaj, 2020)
In mammalian cell culture, the presence of LPS can disrupt experimental integrity by stimulating the release of pro-inflammatory cytokines , inhibiting cell proliferation and transfection efficiency, and, in some cases, inducing apoptosis . In in vivo studies, endotoxins may trigger endotoxic shock and provoke intense immune responses, thereby compromising both safety and data reliability.
To overcome these challenges, Creative BioMart has developed an endotoxin-free E. coli expression platform. The engineered strains retain viability, robustness, and protein expression levels comparable to wild-type strains, but are genetically modified to eliminate the lipid A component of LPS. Through the introduction of suppressor mutations, these strains synthesize a non-toxic LPS analog, while additional genetic modifications ensure stability by preventing reversion to the toxic phenotype.
Service Overview–Endotoxin-Free Protein Production
Service Procedure

Service Details
|
Item |
Details |
|---|---|
|
Expression System |
Our proprietary E. coli strains are genetically engineered to lack lipid A, the endotoxic component of LPS. These strains exhibit normal growth rates, high OD600, and expression yields comparable to wild-type strains—without triggering immune responses. |
|
Production Scale |
We offer scalable production options, from milligram-level for research to multi-gram quantities for preclinical studies. Our expression and fermentation systems are designed for consistency and efficiency across production volumes. |
|
Endotoxin Levels |
Proteins expressed using our system typically fall below 0.1 EU/μg, with further reduction available upon request. This eliminates the need for labor-intensive endotoxin removal and supports direct use in in vivo and cell-based applications. |
|
Protein Types Supported |
We have successfully expressed a wide variety of proteins and oligonucleotide-binding domains, including enzymes, cytokines , antibody fragments, and fluorescent proteins that traditionally express well in E. coli . |
Analytical & Release Panel
-
Identity
Intact-mass LC-MS, peptide mapping
-
Purity
SDS-PAGE, SEC-MALS, RP-HPLC
-
Endotoxin
LAL kinetic chromogenic + rFC confirmatory
-
Bioburden & Sterility
USP <71>
-
Host-Cell Proteins
CHO HCP 2D-DIGE or ELISA < 10 ppm
-
Functional Assay
Customer-supplied or in-house (SPR, enzymatic, cell-based)
-
Aggregation
DLS, AUC, FTIR
-
Glycoanalysis (if mammalian)
2-AB glycan mapping, sialic acid quantitation
Expression Formats
- Tag-free, His-tag, Strep-tag, Fc, GST , Avi-tag , SUMO , MBP
- Site-specific biotinylation (AviTag + BirA)
- Disulfide-rich proteins produced in oxidative cytoplasm (trxB/gor strains) or secreted to periplasm
- Inclusion-body refolding with oxidative/redox buffers and endotoxin stripping
Regulatory & Quality Certifications
- ISO 9001:2015, ISO 13485, cGMP compliant
- Animal-component-free statement & TSE/BSE free
- USP <85> LAL validation package provided
Why Choose Creative BioMart for Endotoxin-Free Protein Production
- True Endotoxin-Free Expression : Unlike traditional systems that require extensive endotoxin removal post-expression, our platform is inherently non-toxic, preventing endotoxins from being introduced in the first place.
- High Yields, Low Risk : Our engineered strains grow robustly and deliver yields equivalent to wild-type E. coli —without compromising solubility or functionality.
- Regulatory-Friendly : Endotoxin-free proteins are ideal for preclinical studies, therapeutic R&D, and GMP transitions, helping you meet global safety standards faster and more efficiently.
- Scalable for Any Stage : Whether you're optimizing a research-grade construct or preparing for large-scale GMP production, our platform adapts seamlessly.
- Fully Customizable Workflow : From solubility tag selection to purification method and final buffer formulation, we tailor every step to meet your protein’s specific needs and application requirements.
- Expert Support at Every Step : Our team of protein engineers and microbiologists offers proactive project updates, detailed troubleshooting, and direct communication to ensure your success from start to finish.
Success Stories in Endotoxin-Free Protein Expression
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Cell-free protein synthesis without endotoxins for cancer therapy
Wilding et al ., 2019. doi:10.1002/biot.201800271
E. coli -based cell-free protein synthesis (CFPS) offers a unique solution for addressing endotoxin contamination in the production of therapeutic proteins using E. coli . Due to its open, cell-free system, CFPS allows for pre-expression endotoxin removal. This could streamline downstream processing and enable on-demand production of therapeutics. This study assessed three endotoxin removal strategies: Triton X-114 extraction, poly-lysine affinity chromatography, and the use of genetically engineered ClearColi lysates. This study presents the first successful use of ClearColi for CFPS, producing high-yield, low-endotoxin proteins. This includes producing crisantaspase, a therapeutic enzyme for acute lymphoblastic leukemia, which demonstrates the platform’s potential for safe, scalable, and efficient therapeutic protein synthesis.
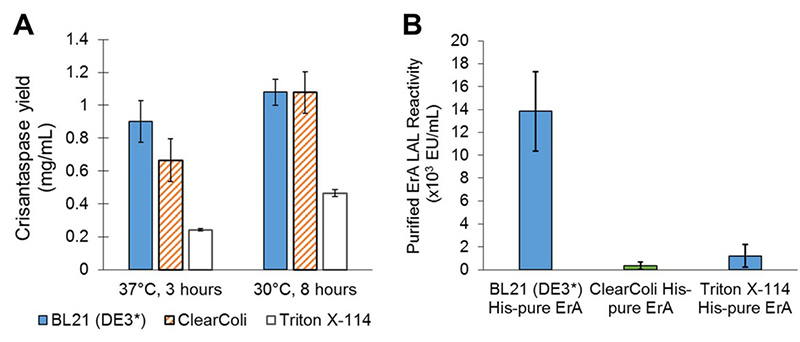
Figure 2. Crisantaspase (ErA) production and endotoxin analysis in reduced-endotoxin CFPS systems. A) ErA yields using BL21(DE3), ClearColi, or Triton X-114-treated extracts at different reaction times. B) Endotoxin levels of His-purified ErA measured by LAL assay across the same extract sources. (Wilding et al ., 2019)
Case 2: Detoxifying E. coli for endotoxin-free production of recombinant proteins
Mamat et al ., 2015. doi:10.1186/s12934-015-0241-5
Removing endotoxins from protein products is technically challenging, costly, and essential to ensure the safety and efficacy of therapeutics, especially for in vivo use. To overcome these limitations, the Team engineered E. coli strains that produce lipid IVA—a non-toxic precursor of lipid A—as the sole LPS-related molecule in their outer membrane. Unlike conventional LPS, lipid IVA does not provoke a strong immune response in humans. Proteins expressed in these strains exhibit extremely low endotoxin levels, eliminating the need for extensive post-expression purification. Growth rates and protein expression remain comparable to wild-type E. coli strains.
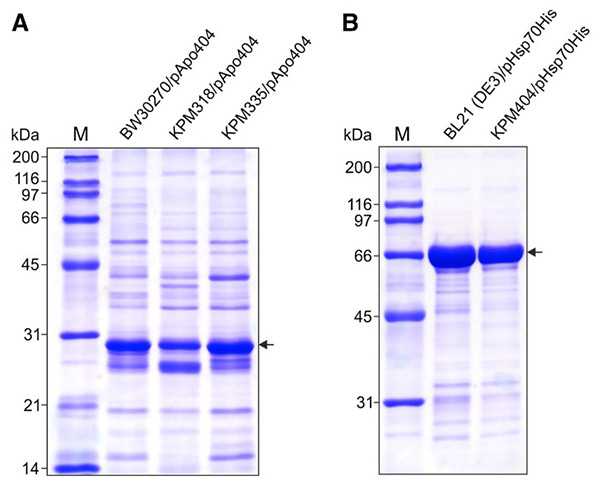
Figure 3. SDS-PAGE gels of ApoA-1 and Hsp70. The proteins were expressed in endotoxin-free derivatives of E. coli strains BW30270 (A) and BL21 (DE3) (B), respectively, and minimally purified using IMAC on HisTrap HP (1 mL) columns. (Mamat et al ., 2015)
Client Feedback on Endotoxin-Free Protein Production Projects
"We needed high-purity cytokines for preclinical in vivo testing, and Creative BioMart delivered exactly that—endotoxin levels under 0.05 EU/μg without any additional purification on our end. Their endotoxin-free E. coli system saved us weeks of cleanup and testing. Excellent communication and reliable results made this a stress-free partnership."
— R&D Director | Biopharmaceutical Company
"We were developing a fusion protein for humanized mouse models, and even trace LPS was derailing our studies. Creative BioMart's engineered E. coli strains produced functionally active protein with virtually no endotoxin. It was ready for injection as-is—no column polishing, no surprises."
— Principal Scientist | Immunotherapy Startup
"Our lab required batch-to-batch consistency for CRISPR-Cas9 experiments, and standard E. coli -expressed proteins kept triggering cell death. Creative BioMart provided recombinant proteins with consistent yields and <0.1 EU/μg, which dramatically improved transfection efficiency and cell viability. Their technical team was helpful and transparent throughout."
— Senior Researcher | Academic Core Facility
"For one of our industrial enzyme projects, we needed gram-scale endotoxin-free production with tight deadlines. Creative BioMart's team delivered on time and exceeded our QC requirements. Their lipid IVA-producing E. coli system is truly a game-changer—no endotoxin removal steps, no compromises on yield or activity."
— Project Manager | Industrial Biotech Company
Frequently Asked Questions–Endotoxin-Free Protein Production
-
Q: What makes your endotoxin-free system different from traditional E. coli expression platforms?
A: Our proprietary E. coli strains are genetically engineered to lack the lipid A component of lipopolysaccharides (LPS), the main driver of endotoxicity. Unlike standard strains, our system is inherently endotoxin-free, eliminating the need for extensive downstream endotoxin removal and reducing risk for in vivo and cell-based applications. -
Q: What are the typical endotoxin levels in proteins produced using your system?
A: Proteins produced with our endotoxin-free E. coli strains typically show endotoxin levels below 0.1 EU/μg, making them ideal for use in sensitive applications such as mammalian cell culture, in vivo studies, and preclinical research. Lower thresholds can be achieved upon request. -
Q: Will switching to your system affect protein yield or solubility?
A: Not at all. Our engineered strains maintain wild-type growth and expression profiles, with comparable yields and solubility. This allows for a seamless transition from conventional E. coli workflows without sacrificing performance. -
Q: Do I still need to perform endotoxin removal steps after purification?
A: In most cases, no. Our system produces protein with ultra-low endotoxin levels directly at the source, minimizing or eliminating the need for downstream endotoxin removal. This saves time, cost, and complexity. -
Q: Can you handle insoluble proteins or inclusion bodies in your system?
A: Yes. We offer refolding protocols and solubility optimization services for proteins that express as inclusion bodies. We also provide fusion tag strategies to enhance solubility when necessary. -
Q: What scale of production do you support?
A: We support production from milligram to multi-gram scales, making our service suitable for early research, pilot studies, or preclinical validation. Scale-up is streamlined, and consistent quality is maintained across volumes. -
Q: What’s your typical turnaround time for an endotoxin-free protein production project?
A: Turnaround times range from 4 to 12 weeks, depending on construct complexity and production scale. We also offer accelerated options for urgent timelines.
Resources
Related Services
- Endotoxin Removal Service
- Bacterial Expression Systems (E. coli / Bacillus)
- High Yield Protein Production
- Large-Scale Protein Production Service
- Custom Membrane Protein Production
- Albumin Fusion Protein Production Services
- Recombinant Antibody Production Services
- Protein Engineering Services
- Fc Fusion Protein Production
- SUMO-tag Protein Production
- Protein Biotinylation
Related Products
References:
- Eckel EF, Ametaj BN. Bacterial endotoxins and their role in periparturient diseases of dairy cows: mucosal vaccine perspectives. Dairy . 2020;1(1):61-90. doi:10.3390/dairy1010006
- Mamat U, Wilke K, Bramhill D, et al . Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. Microb Cell Fact . 2015;14(1):57. doi:10.1186/s12934-015-0241-5
- Wilding KM, Hunt JP, Wilkerson JW, et al . Endotoxin-free E. coli- based cell-free protein synthesis: pre-expression endotoxin removal approaches for on-demand cancer therapeutic production. Biotechnol J . 2019;14(3):1800271. doi:10.1002/biot.201800271
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

