GMP Stable Cell Line Development Platform
Background of GMP-Compliant Stable Cell Line Development in Biopharmaceutical Production
Stable cell line development is a critical process in the production of biologics, including therapeutic proteins, monoclonal antibodies, and vaccines. In this context, a "stable" cell line refers to a population of cells that has been genetically engineered to continuously express a desired product over multiple generations. When intended for clinical or commercial manufacturing, this process must be conducted under Good Manufacturing Practice (GMP) guidelines to ensure product quality, safety, and regulatory compliance.
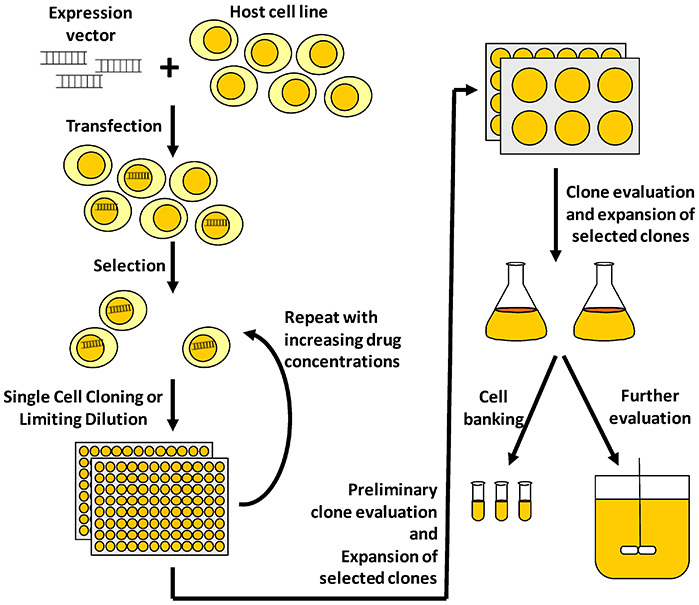
Figure 1. Illustration of a typical process to develop a mammalian cell line for recombinant protein manufacturing. (Lai et al ., 2013)
The extracellular matrix (ECM) is the scaffolding of life—providing structural support, biochemical cues, and dynamic regulation of cell behavior. Matrix proteins like collagen are central to tissue architecture, fibrosis, cancer progression, wound healing, and organ development. Accurate quantification of matrix proteins is essential to study ECM remodeling, monitor fibrosis, validate drug targets, and characterize engineered tissues. That’s where our matrix protein assay suite comes in—targeted, quantitative, and publication-ready.
At Creative BioMart , our Matrix Protein Assay Services offer high-sensitivity, reproducible quantification of total and soluble collagen, hydroxyproline (a key collagen component), and total protein from tissue, cell culture, or engineered constructs. With robust platforms and expert execution, we support studies in fibrosis, tissue engineering, oncology, and regenerative medicine. Whether you're quantifying matrix accumulation or normalizing ECM protein content, our assays are designed for precision and speed.
Our Services for GMP Stable Cell Line Development and Optimization
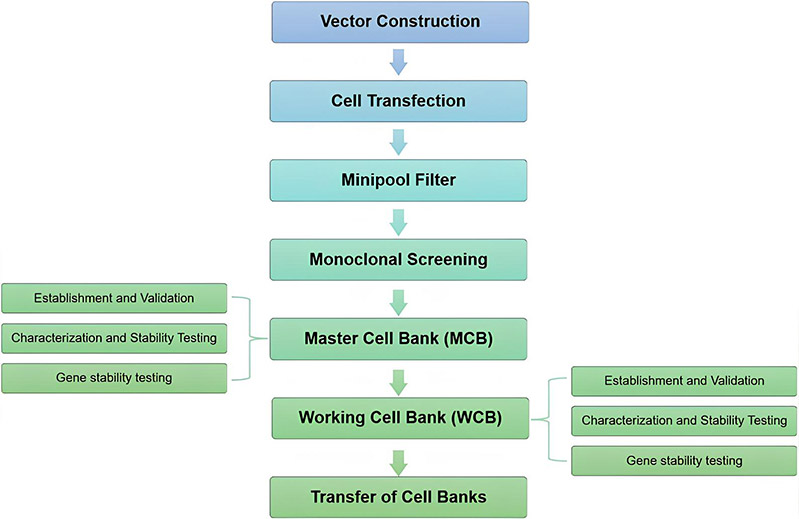
Service Highlights
- Multiple Host Cell Lines : We offer comprehensive vector construction services, including codon optimization, gene synthesis, and vector assembly. Our platform supports a wide range of plasmid backbones and selection systems, with customizable screening strategies. We optimize plasmid regulatory elements and exogenous gene selection to ensure high expression and functional performance across various host cell lines.
- Expertise in Stable Transfection : Building on extensive experience in transient expression systems, we develop high-performance stable cell lines through a systematic evaluation of transient transfection results. This strategic approach enhances success rates and accelerates stable line generation with optimal productivity.
- Robust and Regulatory-Ready Workflow : We ensure all host cells are sourced with full traceability and comply with regulatory expectations. Our development process adheres to stringent standards for data integrity, authenticity, and documentation—offering strong support for clients navigating IND and other regulatory submissions.
- High-Throughput Automation : Equipped with advanced automation systems, our platform enables high-throughput clone screening and precise selection of high-yielding cell lines. This accelerates development timelines while maintaining consistency, efficiency, and data-driven decision-making.
Why Choose Our GMP Platform for Stable Cell Line Development
- Produced under GMP Conditions : All cell line development activities are carried out in strict accordance with GMP guidelines, ensuring regulatory compliance, data integrity, and quality assurance throughout the process.
- Flexible System Options : We support multiple expression systems and host cell platforms, providing tailored solutions that align with your product type, yield goals, and regulatory needs.
- Traceable Host Cell Origins : All host cell lines are sourced with complete documentation and traceability, minimizing regulatory risk and ensuring confidence in cell line lineage.
- Advanced Equipment & Expert Team : Our facility is equipped with high-throughput automation and cutting-edge analytical tools, operated by a skilled technical team with deep expertise in cell line engineering.
- Proven High-Yield Development : With extensive experience in developing stable, high-producing clones, we help shorten timelines and reduce production costs while meeting performance targets.
- One-Stop, Cost-Effective Solution : From vector design to cell banking, we provide an end-to-end platform that integrates speed, scalability, and regulatory readiness—streamlining your path to the clinic or market.
Case Study
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: GMP-compliant production of tumor-lysate-pulsed dendritic cells
Eyrich et al. , 2014. doi:10.1016/j.jcyt.2014.02.017
This study addresses a key challenge in dendritic cell (DC) vaccination: the need for standardized, GMP-compatible production protocols. Researchers successfully transferred an open DC manufacturing method into a closed, GMP-compliant system. The protocol standardized all critical steps, including tumor lysate preparation, monocyte isolation, DC culture, and cryopreservation. Tumor lysate was mechanically homogenized and confirmed avital, yielding consistent protein levels. Monocytes were isolated via elutriation or CD14 selection, with equivalent outcomes.
DCs were differentiated in Teflon bags using specific medium with IL-4 and GM-CSF, then matured with TNF-α and IL-1β after tumor lysate pulsing. The resulting DCs exhibited strong upregulation of maturation markers and retained functionality, including chemokine-directed migration and T-cell activation. Post-cryopreservation stability was confirmed. Clinical comparison in 146 patients showed that GMP-grade DCs were not inferior to those produced via traditional methods. This validated protocol offers a robust foundation for harmonized DC vaccine production and supports future multi-center clinical trials.
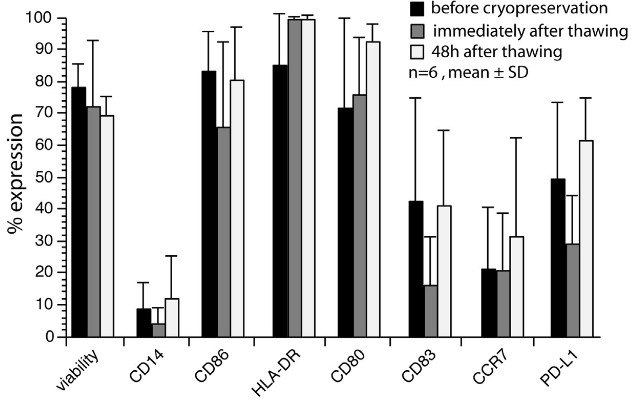
Figure 2. Phenotype and function of cryopreserved and thawed DCs. (Eyrich et al., 2014)
Case 2: cGMP production and characterization of Plasmodium falciparum RH5.1 vaccine in Drosophila S2 cells
Jin et al. , 2018. doi:10.1038/s41541-018-0071-7
Plasmodium falciparum RH5 (PfRH5) is a promising blood-stage malaria vaccine target. To support clinical trials, a full-length PfRH5 protein vaccine, RH5.1, was produced under cGMP using the ExpreS2 platform based on Drosophila S2 cells. A high-yielding monoclonal S2 cell line was developed and used to create a master cell bank. The RH5.1 protein was purified via C-tag affinity and size exclusion chromatography, followed by virus-reduction filtration, yielding over 400 mg of highly pure product. Quality control confirmed the vaccine met all required specifications for identity, sterility, and purity. RH5.1 remained stable for over 18 months at −80 °C. When formulated with the AS01B adjuvant, the protein showed no adverse effects on the liposomal formulation and maintained stability suitable for clinical use. In mice, RH5.1/AS01B induced strong antibody responses that inhibited P. falciparum growth in vitro . These results supported its advancement to Phase I/IIa trials and demonstrate the platform’s suitability for producing complex recombinant vaccines.
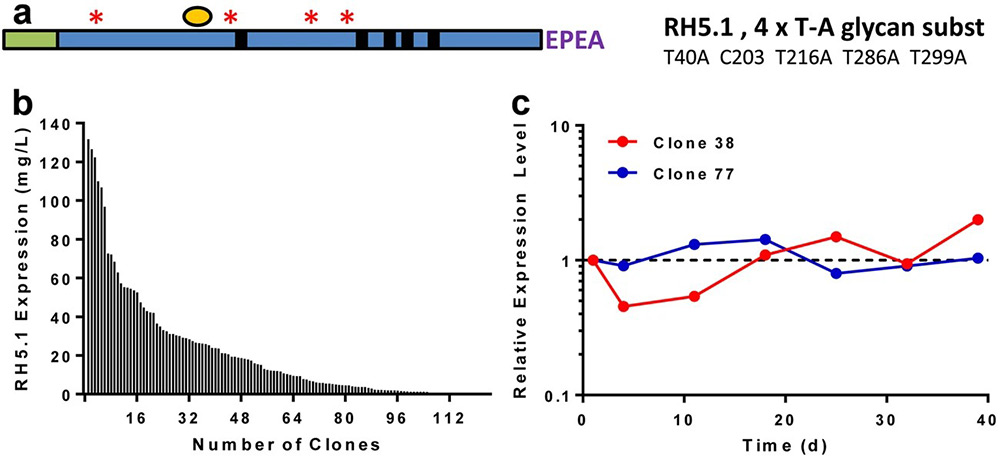
Figure 3. RH5.1 protein vaccine monoclonal stable S2 cell line generation. a Schematic of RH5.1 encoding from the N-terminus. b Expression levels of 124 single clones were measured by ELISA. c Stability of 37 clones was assessed over 73 days. (Jin et al. , 2018)
What Clients Say About Our GMP Stable Cell Line Solutions
"We partnered with Creative BioMart to develop a CHO-based stable cell line for large-scale monoclonal antibody production. Their team demonstrated a deep understanding of vector design and regulatory expectations. The high-producing clone they delivered not only exceeded our titer requirements but also passed our internal stability criteria with ease. Their GMP-compliant workflow gave our regulatory team confidence from day one."
— Director of Biologics R&D | Global Pharmaceutical Company
"Our timeline was tight, and we needed a high-yield stable cell line expressing a bispecific antibody construct. Creative BioMart stood out with their flexible system options and rapid clone screening capabilities. Their advanced automation significantly reduced the timeline, and the resulting cell line scaled beautifully in our fed-batch processes. We appreciated the transparent communication and tech transfer package as well."
— Head of Process Development | Mid-Sized Biotech Company
"We were developing a novel Fc-fusion protein and needed a stable cell line for early tox studies and future GMP production. Creative BioMart provided a one-stop solution—from codon optimization and vector construction to GMP-grade cell banking. Their ability to troubleshoot during transfection and expression bottlenecks made a huge difference. The team was responsive, technically sharp, and fully committed to our success."
— VP of Preclinical Development | Emerging Immunotherapy Startup
"As part of a translational research project, we needed a traceable and stable HEK293 cell line for secreting a therapeutic enzyme. Creative BioMart’s team guided us through every stage, ensuring the line met both academic rigor and industrial standards. The documentation quality was impressive, and we’ve since used the cell line across multiple research collaborations with zero issues."
— Senior Scientist | Academic Research Institute
FAQs on GMP Stable Cell Line Development
-
Q: What host cell lines do you support for GMP stable cell line development?
A: We accept frozen or fresh tissue, paraffin sections, cell lysates, culture supernatants, and even hydrogel scaffolds. -
Q: How do you ensure compliance with GMP standards during cell line development?
A: All our processes follow strict GMP guidelines. From cell banking to clone selection and documentation, every step is performed with full traceability, data integrity, and quality assurance. We also provide comprehensive documentation packages to support regulatory submissions. -
Q: Can you help optimize expression for difficult-to-express proteins?
A: Yes. Our team has extensive experience optimizing vector elements, codon usage, and transfection strategies to boost expression—even for complex constructs like bispecific antibodies or Fc-fusion proteins. We use data from transient expression studies to inform stable line development, improving success rates. -
Q: How long does it typically take to generate a GMP-ready stable cell line?
A: Timelines vary depending on the complexity of the project, but with our advanced automation and high-throughput screening systems, we typically deliver high-yield, stable clones in as little as 3–5 months. Accelerated timelines are available for early-phase programs. -
Q: What kind of testing and analytics do you provide to characterize the cell line?
A: We offer a comprehensive suite of analytical services including productivity assays (e.g., ELISA, BLI), genetic stability (qPCR, STR profiling), and full characterization of critical quality attributes via chromatography and mass spectrometry. This ensures regulatory readiness and downstream success. -
Q: Do you provide GMP-compliant Master and Working Cell Banks?
A: Absolutely. We produce and qualify both Master and Working Cell Banks under GMP conditions, complete with sterility, identity, viability, and adventitious agent testing. Our controlled-rate freezing and cryo storage systems ensure long-term security and compliance. -
Q: Is this a one-stop service, or do I need to work with multiple vendors?
A: We offer a truly end-to-end, one-stop solution—from gene synthesis and vector design through to GMP cell banking and documentation. This integrated approach saves time, reduces cost, and simplifies project management for our clients.
Resources
Related Services
- Stable Cell Line Services
- Cell Line Development and Membrane Preparation
- Protein Expression and Purification Services
- Bacterial Expression Systems (E. coli / Bacillus)
- Mammalian Expression Systems
- Yeast Expression Systems
- Baculovirus-Insect Cell Expression
- Cell-Free In Vitro Protein Expression
- Downstream Processing Development
- High-Throughput Screening
- High-throughput Protein Production
Related Products
References:
- Eyrich M, Schreiber SC, Rachor J, et al . Development and validation of a fully GMP-compliant production process of autologous, tumor-lysate-pulsed dendritic cells. Cytotherapy . 2014;16(7):946-964. doi:10.1016/j.jcyt.2014.02.017
- Jin J, Tarrant RD, Bolam EJ, et al . Production, quality control, stability, and potency of cGMP-produced Plasmodium falciparum RH5.1 protein vaccine expressed in Drosophila S2 cells. npj Vaccines . 2018;3(1):32. doi:10.1038/s41541-018-0071-7
- Lai T, Yang Y, Ng S. Advances in mammalian cell line development technologies for recombinant protein production. Pharmaceuticals . 2013;6(5):579-603. doi:10.3390/ph6050579
- Schulz TC, Young HY, Agulnick AD, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. Lynn FC, ed. PLoS ONE . 2012;7(5):e37004. doi:10.1371/journal.pone.0037004
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

