Nicotiana tabacum Specific Expression Platform
Creative BioMart offers a specialized Nicotiana tabacum Expression Platform for custom recombinant protein production using plant-based, animal component-free processes. This transient expression system leverages non-transgenic tobacco plants to rapidly generate high yields of structurally complex proteins with disulfide bonds and essential post-translational modifications. Ideal for pharmaceutical and industrial use, this method provides a safe, scalable, and cost-effective alternative to traditional microbial and mammalian expression systems. Our strict animal-free protocols and streamlined purification workflows ensure high-purity, contamination-free proteins for your specific applications.

Background: Why Choose Plant-Based Recombinant Protein Expression

The demand for safer, more economical, and scalable protein expression platforms has led to growing interest in plant molecular farming. Among various plant species, Nicotiana tabacum (tobacco) has emerged as the leading host due to its:
- High biomass yield
- Low alkaloid content (safer for downstream processing)
- Compatibility with transient expression systems
- Capability for complex post-translational modifications
Compared to traditional systems ( bacteria, insect, or mammalian cells ), plants are free from mammalian pathogens, have minimal endotoxin levels, and require less expensive equipment and media. These characteristics make plant-based systems particularly attractive for pharmaceutical-grade recombinant protein production.
The process of expressing proteins using the Nicotiana tabacum expression platform involves cultivating tobacco plants, introducing a specific expression vector for genetic modification, activating promoters for mRNA synthesis, translating mRNA into proteins, and accumulating proteins within plant tissues. This is followed by harvesting, purifying, and processing the proteins, ultimately yielding a refined product ready for diverse applications.
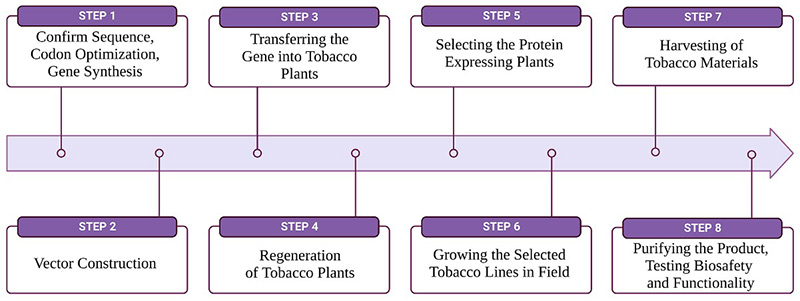
Figure 1. Process of tobacco-based protein expression. (Naeem et al., 2024)
Creative BioMart’s dedicated animal component-free process (ACFP) enhances safety, especially for therapeutic applications, and aligns with regulatory requirements for cGMP manufacturing. Our Nicotiana tabacum expression platform is the culmination of decades of innovation in protein expression and purification.
Service Overview: Customized Plant-Based Protein Expression
Service Procedure
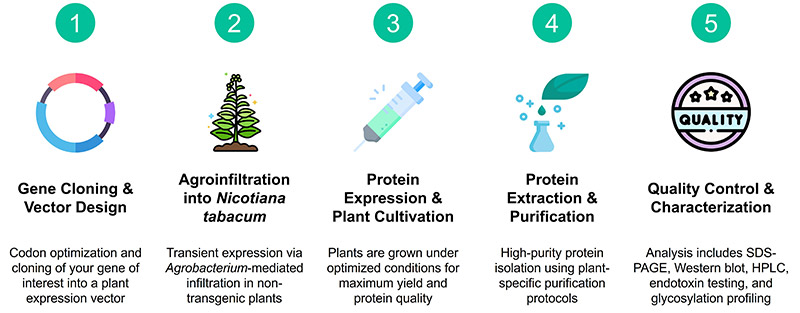
Service Details
-
Host System
Non-transgenic Nicotiana tabacum
-
Expression Method
Transient expression
-
Production Scale
From milligram research-grade to gram-scale industrial quantities
-
Customization
Protein tags, formulation, buffer conditions, QC criteria tailored to your project
-
Animal-Free Certification
Confirmed ACFP materials and SOPs used throughout
Advantages of Using Our Nicotiana tabacum Expression Services
- Completely Animal-Free Workflow: From media to labware, all materials are certified animal-component-free, ideal for regulatory compliance and therapeutic applications.
- Scalable & Cost-Effective: Plant cultivation is inexpensive and easily scalable for industrial-level production.
- Safe & Contaminant-Free: Free from mammalian pathogens and minimal endotoxin risk—suitable for sensitive applications.
- Supports Post-Translational Modifications (PTMs): Plants provide basic PTMs, including disulfide bond formation, for biologically active proteins.
- High Transient Expression Yields: Nic. tabacum delivers rapid, high-yield protein expression, making it ideal for time-sensitive research or preclinical studies.
- Simplified Downstream Processing: Lower impurity levels and reduced alkaloid content simplify purification workflows and improve product quality.
Successful Applications of Nicotiana tabacum Platforms in Biopharmaceutical Development
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Expression of recombinant human Apolipoprotein A-IMilano in Nicotiana tabacum
Zhao et al., 2023. doi:10.1186/s40643-023-00623-w
Apolipoprotein A-IMilano (Apo A-IMilano) is a natural mutant protein with strong potential for treating atherosclerosis by clearing arterial thrombus without notable toxicity. Due to inefficiencies in traditional biopharma platforms, researchers explored Nicotiana tabacum as a novel host for Apo A-IMilano production. Using a modified Agrobacterium-mediated transformation, the gene was inserted into N. tabacum with a Flag/His6/GFP tag. Expression was confirmed via PCR, RT-qPCR, and western blot. The plant-expressed protein reached 0.05 mg/g leaf weight with 90.58% purity and maintained functional lipid-binding activity. This method enables scalable, cost-effective production, supporting the clinical development of Apo A-IMilano.
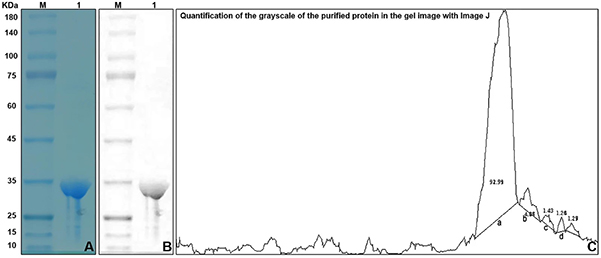
Figure 2. Representative of purification of Flag–Apo A-IMilano protein by SDS–PAGE gel. (Zhao et al., 2023)
Case 2: Production of biologically active hFGFb using Nicotiana tabacum transplastomic plants
Müller et al., 2024. doi:10.1007/s00425-024-04456-5
Researchers successfully engineered transplastomic Nicotiana tabacum lines to express human Basic Fibroblast Growth Factor (hFGFb) within chloroplasts, achieving high-yield production of biologically active recombinant protein. This study highlights the potential of plant-based systems—particularly plastid genome transformation—as efficient, cost-effective platforms for producing high-value human proteins. Two stable, homoplasmic lines accumulated hFGFb in tobacco leaves, and the purified protein retained its functionality by stimulating proliferation in HEK293T cells. These findings support the use of tobacco chloroplasts as scalable biofactories, especially for applications like animal cell culture and regenerative medicine, where recombinant growth factors such as hFGFb are in high demand.
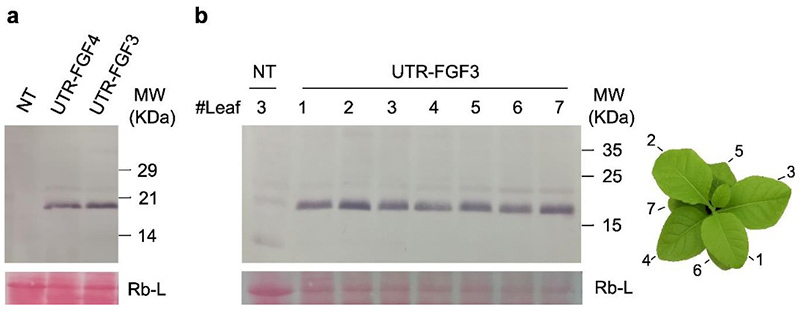
Figure 3. Western blot showing rhFGFb accumulation in UTR-FGF transplastomic lines (a) and across leaf developmental stages (b). Anti-HIS antibody used; Rb-L serves as loading control. (Müller et al., 2024)
Feedback from Researchers Using Our Nicotiana tabacum Protein Expression Systems
"We approached Creative BioMart to express a fusion protein with complex disulfide bonds for an immunotherapy program. Traditional CHO systems were proving slow and costly. The Nicotiana tabacum platform delivered surprisingly high yields in just weeks—with excellent purity and post-translational fidelity. The animal-free aspect was a bonus for our regulatory path. It exceeded our expectations."
— Senior Scientist | Biologics Division, Mid-sized Pharmaceutical Company
"We needed scalable expression of a plant-based antigen candidate for a preclinical trial. Creative BioMart’s tobacco expression system gave us rapid access to gram-scale protein, and the team worked closely with us to optimize glycosylation patterns and stability. The project timeline was tight, but they delivered. Their service was efficient, collaborative, and scientifically sound."
— Director of R&D | Global Vaccine Developer
"Our lab needed a fast and affordable way to produce a cytokine variant for receptor-binding studies. The transient expression in Nicotiana tabacum worked beautifully. The protein was functional and properly folded with minimal downstream challenges."
— Principal Investigator | Academic Research Institute
"Creative BioMart helped us express a multi-domain enzyme in tobacco plants for early-stage screening. Their animal-free, plant-based system was surprisingly robust, and we appreciated their attention to project customization. The downstream purification was straightforward, and endotoxin levels were impressively low—perfect for our proof-of-concept work."
— Head of Protein Engineering | Biotech Startup
Common Questions About Nicotiana tabacum –Based Protein Expression and Production
-
Q: What types of proteins can be expressed using the Nicotiana tabacum platform?
A: Our platform is well-suited for a wide range of recombinant proteins, including enzymes, antibodies, cytokines, vaccine candidates, and fusion proteins. It supports proper folding, disulfide bond formation, and certain post-translational modifications necessary for bioactivity and stability. -
Q: Is the expression system animal-component free?
A: Yes. Our Nicotiana tabacum expression platform is entirely animal-component free. All raw materials, labware, and purification processes are verified ACF, minimizing risk of contamination from mammalian pathogens and aligning with strict regulatory and ethical standards. -
Q: How does this system compare to mammalian or microbial expression platforms?
A: Compared to mammalian or microbial systems, our plant-based platform offers:- Lower production costs
- Rapid, high-yield transient expression
- Minimal endotoxin levels
- Scalable production via simple plant cultivation
- Post-translational modification capability, unlike many microbial hosts
-
Q: Can this platform support GMP-compliant production?
A: Yes. Plant-based systems, including Nicotiana tabacum , can be adapted for production under current Good Manufacturing Practice (cGMP) conditions, making them suitable for pharmaceutical-grade biologics. -
Q: How quickly can I receive my expressed protein?
A: Turnaround times vary by protein complexity and quantity, but due to the speed of transient expression, initial protein yields can often be delivered within 4–8 weeks of project initiation. -
Q: Do you offer customized services for unique protein expression needs?
A: Absolutely. Every project is tailored—from codon optimization and vector design to expression conditions and purification strategy. We collaborate closely with you to meet specific functional and regulatory requirements.
Resources
Related Services
- Mammalian Expression Systems
- Yeast Expression Systems
- Baculovirus-Insect Cell Expression
- Bacterial Expression Systems (E. coli / Bacillus)
- Cell-Free In Vitro Protein Expression
- Protein Expression and Purification Services
- Downstream Processing Development
- Endotoxin Removal Service
- Endotoxin-free Protein Production
Related Products
References:
- Müller C, Budnik N, Mirkin FG, et al. Production of biologically active human basic fibroblast growth factor (hFGFB) using Nicotiana tabacum transplastomic plants. Planta. 2024;260(1):28. doi:10.1007/s00425-024-04456-5
- Naeem M, Han R, Ahmad N, Zhao W, Zhao L. Tobacco as green bioreactor for therapeutic protein production: latest breakthroughs and optimization strategies. Plant Growth Regul. 2024;103(2):227-241. doi:10.1007/s10725-023-01106-w
- Zhao W, Zhou LY, Kong J, et al. Expression of recombinant human apolipoprotein A-Imilano in Nicotiana tabacum. Bioresour Bioprocess. 2023;10(1):4. doi:10.1186/s40643-023-00623-w
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

