Organelle Protein Extraction
Accurate characterization of organelle-specific proteins is essential for understanding the dynamic organization and functional responses of cells. Creative BioMart provides comprehensive Organelle Protein Extraction Services designed to isolate, enrich, and analyze proteins from subcellular compartments with exceptional clarity and reproducibility. Using advanced fractionation strategies, optimized detergent-based extraction workflows, and state-of-the-art analytical methods, we deliver high-quality protein samples suitable for downstream proteomic, metabolomic, and functional studies. With decades of expertise in subcellular biology and high-resolution protein analysis, we support research across plant, animal, microbial, and clinical samples, enabling clients to investigate organelle-specific mechanisms underlying cellular physiology, stress response, and disease progression.

Why Is Organelle Protein Extraction Necessary
In recent years, organelle proteomics has emerged as a powerful tool for exploring cell biology at previously inaccessible resolution. For example, studies on the proteomes of major organelles in plant cells have significantly advanced our knowledge of how crops respond to abiotic stresses, such as drought, salinity, and flooding. Similarly, mitochondrial proteome analysis across diverse cell types has become instrumental in cancer research, metabolic disease studies, and developmental biology.
Typical organelle protein extraction begins with physical fractionation through differential or density-gradient centrifugation, producing enriched subproteomic fractions such as cytosolic, membrane-associated, organelle, nuclear, and cytoskeletal proteins. Follow-up extraction with optimized buffers ensures high purity and minimal cross-contamination—critical for reliable downstream studies. Extracted proteins can then be analyzed using advanced platforms, including 2-DE, LC-MS/MS, SDS-PAGE, MALDI-TOF MS, and gel-free quantitative methods. Together, these technologies enable comprehensive characterization of organelle proteomes and support detailed biological and functional insights.
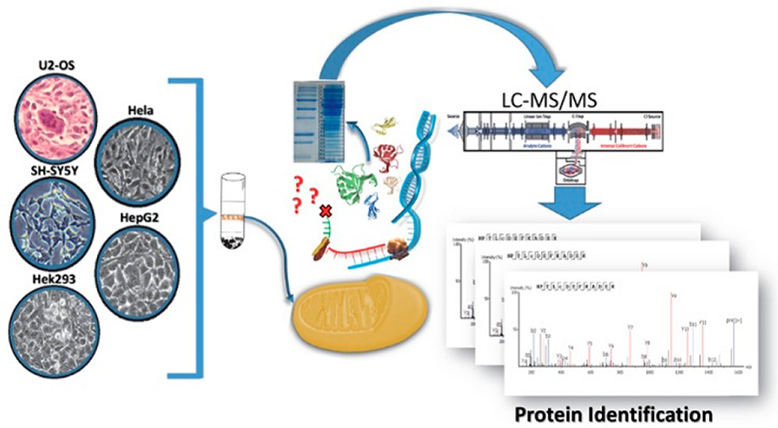
Figure 1. An example for extraction and identification of mitochondrial proteins. (Ronci et al., 2018)
Organelle Protein Extraction: What We Offer
Creative BioMart delivers end-to-end organelle protein extraction services with full customization to match the biological system, sample type, and research objective. Our offerings include:
-
Organelle Isolation and Purification
High-quality, high-purity isolation from diverse sources including:
- Mitochondria
- Nucleus
- Chloroplasts
- Cell wall fractions
- Plasma membrane
- Peroxisomes
- Endoplasmic reticulum or Golgi (optional customized workflows)
We optimize every step—buffer formulation, gradient selection, and centrifugation parameters—to ensure minimal cross-contamination.
-
Organelle-Specific Protein Extraction
Sequential extraction protocols enable recovery of distinct subcellular protein pools, including:
- Cytosolic and soluble proteins
- Membrane-bound and organellar proteins
- Nuclear proteins
- Cytoskeletal filament-associated proteins
-
Protein Separation and Fractionation
Our analytical platforms support diverse separation strategies:
- 2-DE
- SDS-PAGE
- Native PAGE
- Chromatographic separation (HPLC, SEC, ion-exchange)
- Gel-free separation for mass spectrometry
-
High-Confidence Protein Identification
Using cutting-edge mass spectrometry platforms:
- LC-MS/MS
- MALDI-TOF MS
- Tandem MS for PTM analysis
-
Metabolome Analysis (Optional)
Parallel profiling of organelle-associated metabolites to complement proteomic findings.
-
Fully Customized Analysis Packages
Including quantitative proteomics, differential expression analysis, and pathway mapping.
Service Workflow
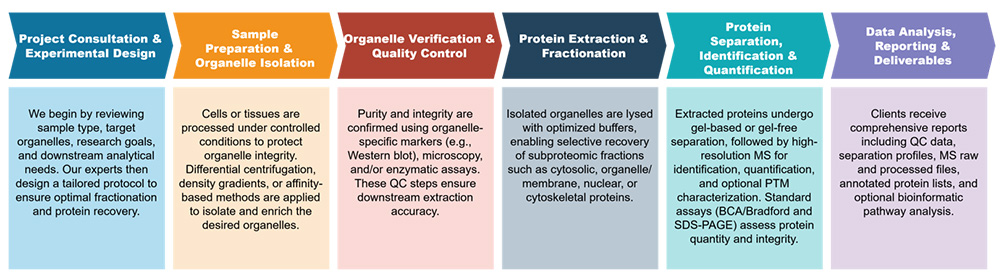
Available Extraction Approaches
Our organelle protein extraction platform leverages advanced centrifugation methodologies to produce clean, high-purity extracts suitable for in-depth proteomic analysis. Depending on sample type and organelle complexity, we may apply:
| Approach |
Specification |
|---|---|
|
Differential Centrifugation |
Ideal for mitochondria, chloroplasts, and nuclei. |
|
Density Gradient Purification |
Using sucrose, Percoll, or OptiPrep gradients to eliminate contaminants and ensure high organelle purity. |
|
Detergent-Assisted Extraction |
Mild or harsh detergents selected based on protein solubility and downstream application. |
|
Sequential Extraction Approaches |
Generating up to four distinct protein fractions:
|
Analytical Flexibility
We support multiple workflows for protein identification:
- Quantitative proteomics (TMT, iTRAQ, LFQ)
- PTM analysis (phosphorylation, ubiquitination, glycosylation)
- Targeted MS for specific pathways or protein families
Sample Compatibility
We routinely process:
- Mammalian cells
- Microbial organisms
- Plant tissues
- Clinical specimens
- Purified cell populations
Apart Why Choose Us
- Decades of Specialized Expertise: With long-standing experience in organelle biology and proteomics, our teams deliver highly reliable data with meticulous precision.
- High-Purity Organelle Isolation: Our optimized centrifugation and gradient strategies minimize cross-contamination and preserve native protein structure.
- Flexible, Customizable Workflows: We tailor extraction buffers, purification steps, and MS methodologies to the specific needs of each project.
- Comprehensive Analytical Capabilities: From separation to MS identification and metabolomics, we offer a fully integrated suite of advanced analytical tools.
- Robust Quality Assurance: Every stage—from organelle enrichment to data reporting—is governed by rigorous QA/QC protocols for maximal reproducibility.
- Support for Complex Samples: Whether handling delicate plant tissues, clinical samples, or stress-exposed organisms, we ensure high-quality results even under challenging conditions.
Organelle Protein Extraction: Case Studies
Case 1: Subcellular protein extraction from human pancreatic cancer tissues
Börner et al., 2009. doi:10.2144/000113090
Proteins are key effectors in cellular systems, making reliable protein analysis essential for distinguishing healthy from diseased tissues. However, their structural complexity, interactions, and instability pose major isolation challenges—especially in protease-rich tissues like the pancreas. Effective extraction requires breaking cell–cell and cell–matrix connections without compromising protein integrity. By comparing multiple approaches, researchers established a robust workflow using tissue slicing followed by cell isolation to achieve reproducible fractionation of cytosolic, membrane/organelle, nuclear, and cytoskeletal proteins. Tissue slices also enable histochemical assessment of sample quality and ensure accurate representation or selective sampling across heterogeneous tumor regions.
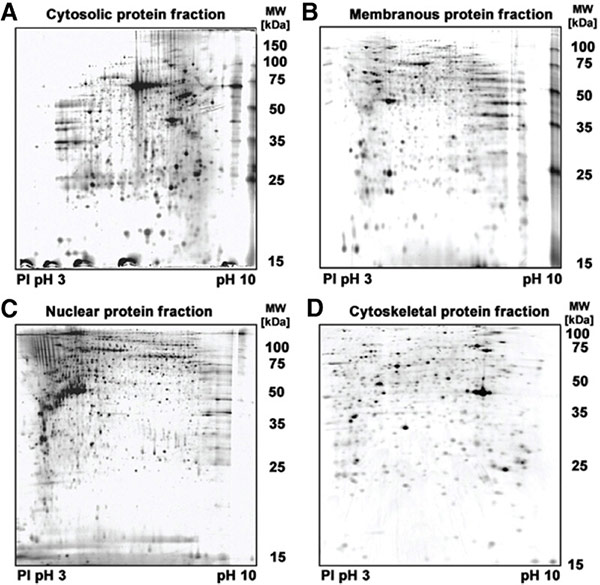
Figure 2. Silver-stained two-dimensional gels of protein fractions. Typical results of cytosolic (A), membranous (B), nuclear (C), and cytoskeletal (D) protein extracts are shown, which were isolated by the cryocut section process from cancerous human pancreas tissue. (Börner et al., 2009)
Case 2: ER proteomic insights into soybean responses to flooding and drought
Wang and Komatsu, 2016. doi:10.1021/acs.jproteome.6b00190
Soybean, though widely cultivated, is vulnerable to flooding and drought, both of which trigger endoplasmic reticulum (ER) stress through the buildup of misfolded proteins. ER proteomics of soybean root tips revealed high-purity ER isolation and notable reductions in ribosomal proteins under both stresses, with flooding showing a greater proportional increase in ribosomes. Protein glycosylation and signaling pathways were broadly affected, while calnexin, PDI-like proteins, and heat shock proteins showed stress-specific declines. Both stresses led to fewer glycoproteins and elevated cytosolic calcium, suggesting disrupted calnexin/calreticulin folding cycles and impaired ER homeostasis during environmental stress.
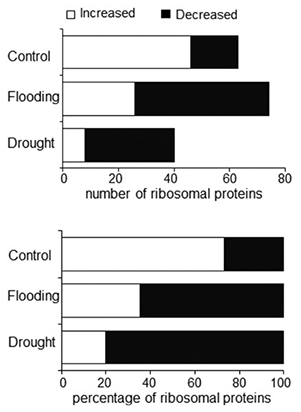
Figure 3. Ribosomal proteins affected by flooding and drought stresses. Two-day-old soybeans were treated without or with flooding and drought for 2 days. The number and percentage of identified ribosomal proteins that increased and decreased in abundance are shown for control and flooding- and drought-stressed seedlings. (Wang and Komatsu, 2016)
Organelle Protein Extraction: Customer Feedback
“We engaged Creative BioMart to isolate mitochondria and nuclei from patient-derived tumor biopsies for a targeted proteomics study. Their team delivered organelle fractions with remarkable purity—confirmed by our own marker analysis—which dramatically improved the signal quality in our LC-MS/MS datasets. The reproducibility across batches was outstanding, enabling us to confidently map compartment-specific protein changes associated with chemotherapy resistance. Their technical expertise saved us weeks of troubleshooting and significantly accelerated our biomarker discovery timeline.”
— Director of Translational Research | Global Pharmaceutical Company
“Our project required chloroplast and ER protein extraction from soybean roots under drought and flooding conditions—traditionally a difficult matrix due to stress-induced protease activity. Creative BioMart not only preserved protein integrity but also delivered subcellular fractions clean enough for downstream glycoproteomics. Their optimized protocols allowed us to identify subtle shifts in organelle-localized folding chaperones that we had never been able to detect before. Their team clearly understands the nuances of plant organelle proteomics.”
— Lead Scientist, Crop Improvement Division | Agricultural Biotechnology Enterprise
“For a project dissecting nuclear–cytoplasmic shuttling of immune regulators, we relied on Creative BioMart’s fractionation services. They generated cytosolic, membrane/organelle, and nuclear extracts with excellent separation efficiency—no detectable cross-contamination in our validation blots. This level of precision allowed us to track stimulus-dependent translocation of transcription factors with confidence. Their responsiveness and detailed QC documentation were exactly what we needed for publication-grade data.”
— Head of Cell Biology | Immunology Research Institute
“Our team needed simultaneous extraction of mitochondria, plasma membrane, and peroxisomal proteins from engineered human cell lines to support a metabolic-engineering pipeline. Creative BioMart provided a seamless, well-structured workflow—from sample receipt to mass-spec-ready extracts. The consistency of their preparations enabled our metabolome and proteome datasets to align perfectly, helping us pinpoint target bottlenecks in our pathway optimization project.”
— Senior Manager, Proteomics & Analytical Sciences | Biotech Startup
Organelle Protein Extraction: Frequently Asked Questions (FAQs)
-
Q: What types of organelles can you isolate with high purity?
A: We routinely isolate mitochondria, nuclei, chloroplasts, ER, peroxisomes, plasma membranes, and cell wall fractions from a wide range of animal, plant, and microbial samples. Our decades of experience in subcellular fractionation ensure high-purity organelle preparations, validated through organelle-specific markers and activity assays. -
Q: Do you work with challenging or protease-rich samples (e.g., pancreatic tissues or stressed plant tissues)?
A: Yes. We have optimized workflows specifically for difficult matrices such as pancreatic tissue, plant roots under abiotic stress, and samples with high protease activity. Our proprietary extraction strategy minimizes protein degradation, maintains organelle integrity, and preserves even low-abundance or stress-responsive proteins. -
Q: Can you provide multiple subcellular protein fractions from a single sample?
A: Absolutely. Using sequential fractionation methods, we can generate cytosolic, membrane/organelle, nuclear, and cytoskeletal protein extracts from a single tissue or cell sample. This allows customers to perform comprehensive subcellular proteomics and track protein translocation events with precision. -
Q: What downstream analyses can you support after extraction?
A: We offer a full suite of analytical services, including 2-DE, SDS-PAGE, LC-MS/MS, MALDI-TOF MS, gel-free proteomics workflows, and metabolomic profiling. This end-to-end integration eliminates the need to ship samples elsewhere and ensures consistent data quality throughout the pipeline. -
Q: How do you ensure reproducibility and purity of organelle fractions?
A: Each project includes rigorous QC steps—such as marker enzyme assays (e.g., NADH cytochrome c reductase for ER purity), immunoblot validation, and contamination profiling—to confirm organelle enrichment and reproducibility across batches. These QC measures are publication-ready and suitable for regulatory documentation. -
Q: Can you handle stress-response or signaling studies requiring subtle protein abundance measurements?
A: Yes. Our refined extraction protocols maintain fragile protein complexes, post-translational modifications, and stress-related chaperones, enabling high-sensitivity detection of dynamic biological changes. This is particularly valuable for studies on ER stress, organelle-localized signaling, and protein-folding pathways. -
Q: Do you offer custom workflows for unique organisms or experimental designs?
A: Certainly. Whether you are working with rare clinical biopsies, engineered cell lines, crop tissues under environmental stress, or difficult subcellular compartments, we tailor buffer systems, centrifugation steps, and purification strategies to match your project's requirements.
Other Resources
Related Services
- Protein Extraction Services
- Nuclear Protein Extraction
- Membrane Protein Extraction
- Cytoplasmic Protein Extraction
Related Products
References:
- Börner A, Warnken U, Schnölzer M, et al. Subcellular protein extraction from human pancreatic cancer tissues. BioTechniques. 2009;46(4):297-304. doi:10.2144/000113090
- Ronci M, Pieroni L, Greco V, et al. Sequential fractionation strategy identifies three missing proteins in the mitochondrial proteome of commonly used cell lines. J Proteome Res. 2018;17(12):4307-4314. doi:10.1021/acs.jproteome.8b00422
- Wang X, Komatsu S. Gel-free/label-free proteomic analysis of endoplasmic reticulum proteins in soybean root tips under flooding and drought stresses. J Proteome Res. 2016;15(7):2211-2227. doi:10.1021/acs.jproteome.6b00190
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

