Over-Expression Lysates
🧪 TNFSF11-001CCL
Source: Human Cells
Species: Cynomolgus
Tag: Non
Conjugation:
Protein Length:

🧪 SEMA4D-001CCL
Source: Human Cells
Species: Cynomolgus
Tag: Non
Conjugation:
Protein Length:

🧪 AMY2A-001CCL
Source: Human Cells
Species: Cynomolgus
Tag: Non
Conjugation:
Protein Length:

🧪 IL18R1-001CCL
Source: Human Cells
Species: Cynomolgus
Tag: Non
Conjugation:
Protein Length:

Background
Overview
Overexpressed cell lysate is a molecular biology tool that is usually a mixture of cells that have been overexpressed by a specific gene and are lytically treated. These lysates contain all the proteins in the cell, including the target overexpressed proteins. This lysate is very useful in protein studies, especially in protein expression, functional analysis, antibody production and validation, and protein interaction studies.
Preparation Process
Choose a suitable host cell line, such as an insect cell or a mammalian cell. It depends on the complexity of the target protein and the required post-translational modifications.
The expression vector containing the target gene is transfected or transformed into the host cell so that it is overexpressed in the cell.
The transformed cells were cultured to expand and overexpress the target protein.
Cells are collected and cleaved to release all proteins within the cell, including overexpressed target proteins.
Advangtages
High expression. It provides high concentration of target protein, which is convenient for biochemical experiment and functional study.
High efficiency. It can quickly obtain a large number of samples and accelerate the experimental process.
Biological correlation. Compared with recombinant proteins synthesized in vitro, the target proteins in overexpressed cell lysates may retain more biological activity and post-translational modifications, and are more suitable for functional studies.
Notes
Background proteins: Lysates may contain a large number of non-target proteins, which may affect the detection and analysis of specific proteins.
Protein stability: During cleavage and storage, the stability of the target protein requires special attention to prevent degradation
Reproducibility: Potential batch variability should be considered, which may affect the repeatability and reliability of the experiment.
Applications
- Protein function Studies: By overexpressing cell lysates, researchers can study the function of a target protein, including its enzyme activity, binding properties, or signaling role.
- Antibody production and validation: The lysate can be used to produce specific antibodies against the target protein and to verify the specificity and potency of the antibody through techniques such as Western Blot.
- Protein interactions: Immunocoprecipitation (Co-IP) experiments using overexpressed cell lysates can identify other proteins that interact with the target protein.
- Protein purification: The lysate can be used as the starting material to purify the target protein, and the target protein can be further purified by affinity chromatography and other methods.
Case Study
Case Study 1: Recombinant Human SMN1 293 Cell Lysate (SMN1-1660HCL)
In recent years, due to the high conductivity, mechanical strength and large surface area of carbon nanomaterials, the integration of carbon nanomaterials into electrochemical sensors has aroused great interest. However, no comparative study on aptamer sensing applications of different carbon nanomaterials has been reported. This study reports a comparative study of the properties of six carbon electrode materials (carbon, graphene (G), graphene oxide (GO), multi-walled carbon nanotubes (MWCNT), single-walled carbon nanotubes (SWCNT), and carbon nanofibers (CNF)) on physical adsorption-prepared hemoglobin A1C (HbA1c) apperomer sensors. Comparison of aptamer adsorption, sensor response and selectivity of different nanomaterials showed that swcnts sensors had better performance. For the HbA1c selectivity experiments, the aptamer-modified electrode was incubated with 20 μL solution of 1 ng/mL of CFTR, SMN, STAT3 and DOCK8 for 30 min. And Voltammetric swcnts aptamer sensors showed high sensitivity and selectivity for the detection of total hemoglobin (tHb) and glycosylated hemoglobin (HbA1c). It is selective for blood proteins such as cystic fibrosis transmembrane conduction regulator (CFTR), survival motor neuron (SMN), cytoplasmic division dedication factor 8 (DOCK8), signal transducer and transcriptional activator 3 (STAT3).
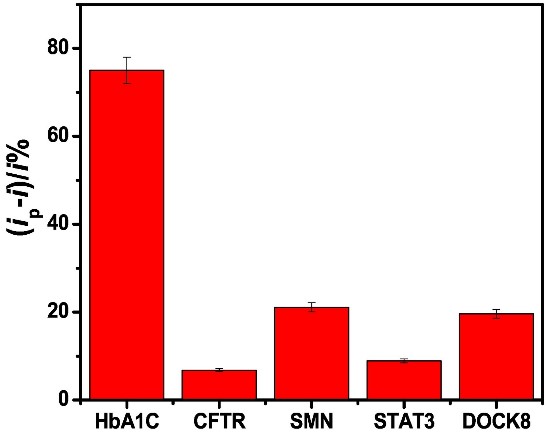
(Shimaa Eissa, 2019)
Fig1. The HbA1c aptamer assay selectivity against 1 ng/mL of HbA1c, CFTR, SMN, STAT3 and DOCK8.
Case Study 2: Recombinant Human ATF1 293 Cell Lysate (ATF1-8632HCL)
Multiple genome-wide studies have identified associations between the outcome of human immunodeficiency virus (HIV) infection and polymorphisms in and around the gene encoding the HIV coreceptor CCR5, but the functional basis of the strongest of these associations, rs1015164A/G, has been unclear. rs1015164 was found to mark a variation in the activation transcription factor 1 binding site, which controls expression of antisense long non-coding RNA (lncRNA) CCR5AS. Knockdown or enhancement of CCR5AS expression leads to corresponding changes in CCR5 expression on CD4+ T cells. CCR5AS interferes with the interaction between the RNA-binding protein Raly and the 3' untranslated region of CCR5, protecting CCR5 messenger RNA from RALy-mediated degradation. Reducing CCR5 expression by inhibiting CCR5AS in vitro can reduce CD4+ T cell infection with CCR5-CCR5-addicted HIV. In electrophoretic mobility shift assay, Lysate from HEK 293 transfected with recombinant human Atf1 was used as a positive control.
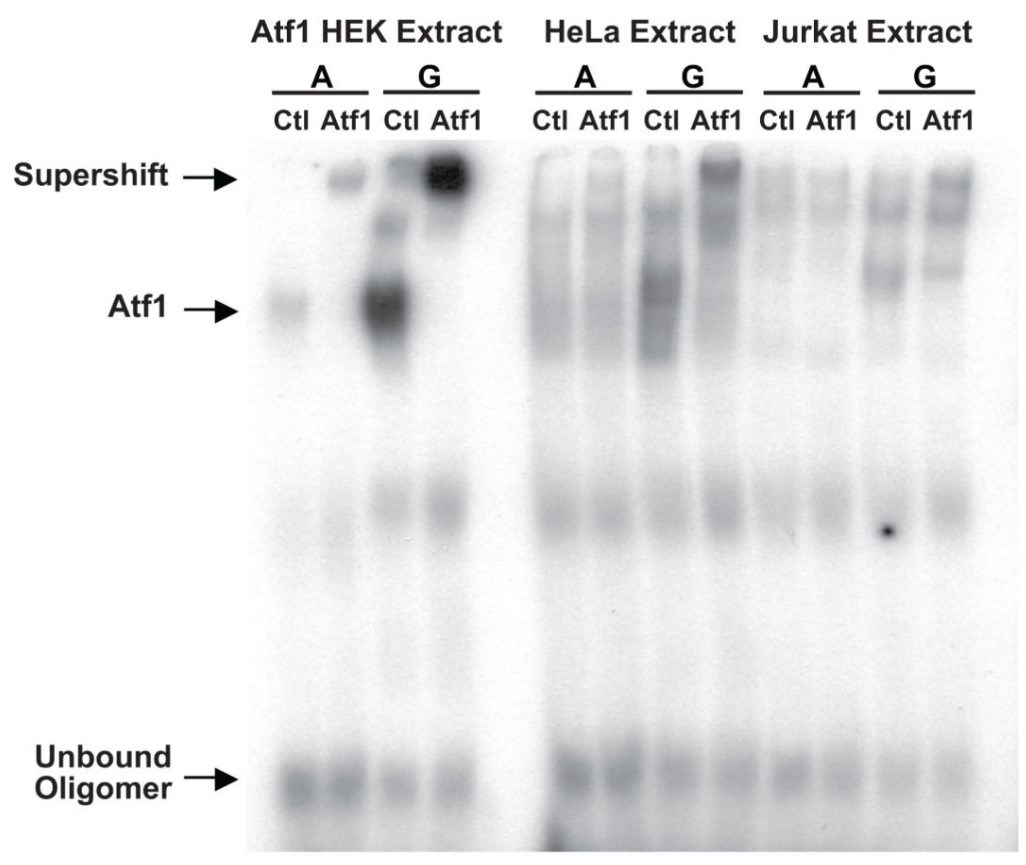
(Shimaa Eissa, 2019)
Fig2. An electrophoretic mobility shift assay (EMSA) was performed with 32P-labelled oligonucleotides containing the core binding sequence for ATF1 containing the A or G variant of rs2027820.
Case Study 3: Recombinant Human SMN1 293 Cell Lysate (SMN1-1660HCL)
High immunoglobulin E syndrome (HIES) is a rare inherited birth defect, and HIESs is inherited through heterozygous or homozygous mutations in the signaling transducer and transcriptional activator 3 (STAT3) or cell division dedication factor 8 (DOCK8) genes, in autosomal dominant and recessive forms, respectively. Therefore, early detection of human DOCK8 and STAT3 protein levels would aid in the early diagnosis of these diseases and thus in the management of the disease. This study presents the development of immunosensors using electrochemical impedance spectroscopy to detect DOCK8 and STAT3 in a label-free format. The immunosensor was prepared by covalently attaching DOCK8 and STAT3 specific antibodies to gold electrode with cysteamine/phenyl diisothiocyanate linker. This detection is achieved by monitoring the change in charge transfer resistance (Rct) after the iron/ferricyanide REDOX pair binds to the surface of the immunosensor. These biosensors are capable of detecting DOCK8 and STAT3 levels with detection limits of 1.2 and 9.0 pg/ml, respectively. The immunosensor was also applied to the detection of DOCK8 in human serum samples, showing a high recovery rate.
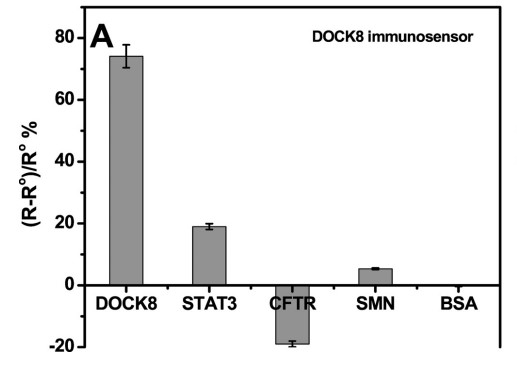
(Shimaa Eissa, 2019)
Fig3. Sensor responses of the DOCK8 immunosensor to 1 ng/mL of BSA, STAT3, CFTR or SMN.
Case Study 4: Recombinant Human SMN1 293 Cell Lysate (SMN1-1660HCL)
Spinal muscular atrophy is an untreatable potentially fatal hereditary disorder caused by loss-of-function mutations in the survival motor neuron (SMN) 1 gene which encodes the SMN protein. There is an urgent unmet need to accurately quantify SMN protein levels for screening and therapeutic monitoring of symptomatic newborn and SMA patients, respectively. Here, the research team developed a voltammetric immunosensor for the sensitive detection of SMN protein based on covalently functionalized carbon nanofiber-modified screen printed electrodes. A comparative study of six different carbon nanomaterial-modified electrodes (carbon, graphene (G), graphene oxide (GO), single wall carbon nanotube (SWCNT), multi-wall carbon nanotube (MWCNT), and carbon nanofiber (CNF)) was performed. The electrochemical characterization and analytical performance of the six immunosensors suggest that carbon nanofiber is a better electrode material for the SMN immunosensor. The voltammetric SMN carbon nanofiber-based immunosensor showed high sensitivity (detection limit of 0.75pg/ml) and selectivity against other proteins such as cystic fibrosis transmembrane conductance regulator (CFTR) and dystrophin (DMD).
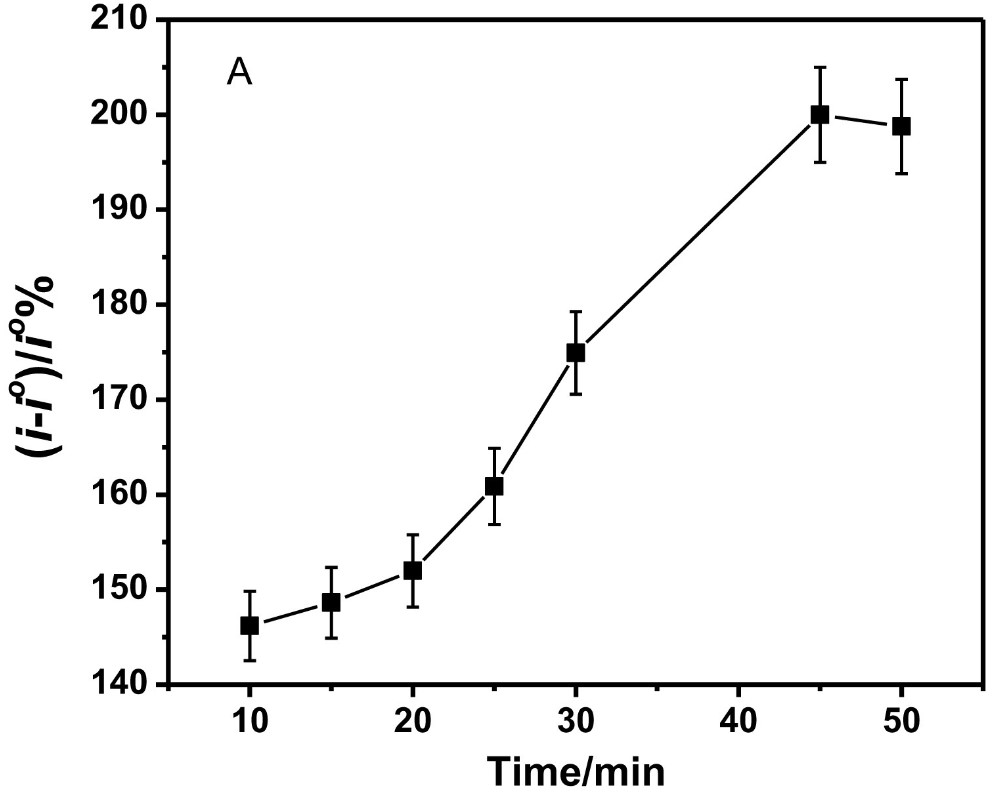
(Shimaa Eissa, 2018)
Fig4. CNF immunosensor response with increasing incubation time of the SMN protein.
Advantages
- Pure and efficient. We use advanced biotechnology to ensure unparalleled purity and activity in each batch, making your test results more accurate and reliable.
- Comprehensive product line. Whether you are looking for a specific label protein, enzyme or expression host, our extensive product range meets your every need, so that your research is no longer limited.
- Custom service. Our team of experts provide personalized services, from gene cloning to protein purification, each step is carefully crafted to meet your unique scientific requirements.
Creative BioMart can provide Over-Expression Lysates that express in different hosts. Our lysates are validated and are available in a variety of normal, tumor and disease characterized tissues. You can also let us know if you have any customized requirements. Please contact us for more product details.
References
- Eissa S, Almusharraf AY, Zourob M. A comparison of the performance of voltammetric aptasensors for glycated haemoglobin on different carbon nanomaterials-modified screen printed electrodes. Mater Sci Eng C Mater Biol Appl. 2019;101:423-430.
- Kulkarni S.; et al. CCR5AS lncRNA variation differentially regulates CCR5, influencing HIV disease outcome [published correction appears in Nat Immunol. 2019 Nov;20(11):1555]. Nat Immunol. 2019;20(7):824-834.
- Eissa, S.; et al. “Development of Impedimetric Immunosensors for the Diagnosis of DOCK8 and STAT3 Related Hyper‐Immunoglobulin E Syndrome.” Electroanalysis. 2018;30:2021-2027.
- Eissa S.; et al. Electrochemical immunosensors for the detection of survival motor neuron (SMN) protein using different carbon nanomaterials-modified electrodes. Biosens Bioelectron. 2018;101:282-289.












