Recombinant Human SMN1 293 Cell Lysate
| Cat.No. : | SMN1-1660HCL |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | HEK293 |
| Tag : | Non |
| Description : | Antigen standard for survival of motor neuron 1, telomeric (SMN1), transcript variant d is a lysate prepared from HEK293T cells transiently transfected with a TrueORF gene-carrying pCMV plasmid and then lysed in RIPA Buffer. Protein concentration was determined using a colorimetric assay. The antigen control carries a C-terminal Myc/DDK tag for detection. |
| Components : | This product includes 3 vials: 1 vial of gene-specific cell lysate, 1 vial of control vector cell lysate, and 1 vial of loading buffer. Each lysate vial contains 0.1 mg lysate in 0.1 ml (1 mg/ml) of RIPA Buffer (50 mM Tris-HCl pH7.5, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1% NP40). The loading buffer vial contains 0.5 ml 2X SDS Loading Buffer (125 mM Tris-Cl, pH6.8, 10% glycerol, 4% SDS, 0.002% Bromophenol blue, 5% beta-mercaptoethanol). |
| Size : | 0.1 mg |
| Storage Instruction : | Store at -80°C. Minimize freeze-thaw cycles. After addition of 2X SDS Loading Buffer, the lysates can be stored at -20°C. Product is guaranteed 6 months from the date of shipment. |
| Applications : | ELISA, WB, IP. WB: Mix equal volume of lysates with 2X SDS Loading Buffer. Boil the mixture for 10 min before loading (for membrane protein lysates, incubate the mixture at room temperature for 30 min). Load 5 ug lysate per lane. |
| Publications : |
|
| Gene Name | SMN1 survival of motor neuron 1, telomeric [ Homo sapiens ] |
| Official Symbol | SMN1 |
| Synonyms | SMN1; survival of motor neuron 1, telomeric; SMA, SMA@, spinal muscular atrophy (Werdnig Hoffmann disease, Kugelberg Welander disease); survival motor neuron protein; BCD541; SMA1; SMA2; SMA3; SMNT; gemin 1; gemin-1; component of gems 1; SMA; SMN; SMA4; SMA@; SMN2; T-BCD541; |
| Gene ID | 6606 |
| mRNA Refseq | NM_000344 |
| Protein Refseq | NP_000335 |
| MIM | 600354 |
| UniProt ID | Q16637 |
| Chromosome Location | 5q13.2 |
| Pathway | Gene Expression, organism-specific biosystem; Metabolism of non-coding RNA, organism-specific biosystem; RNA transport, organism-specific biosystem; RNA transport, conserved biosystem; Survival motor neuron (SMN) complex, organism-specific biosystem; snRNP Assembly, organism-specific biosystem; |
| ◆ Recombinant Proteins | ||
| SMN1-947C | Recombinant Cynomolgus SMN1 Protein, His-tagged | +Inquiry |
| SMN1-001H | Recombinant Human SMN1 Protein, GST-tagged | +Inquiry |
| SMN1-6315H | Recombinant Human SMN1 Protein (Ala2-Asn294), His tagged | +Inquiry |
| SMN1-30H | Recombinant Human SMN1 Protein, Myc/DDK-tagged | +Inquiry |
| SMN1-506HF | Recombinant Full Length Human SMN1 Protein | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| SMN1-1660HCL | Recombinant Human SMN1 293 Cell Lysate | +Inquiry |
Case 1: Eissa S, et al. Biosens Bioelectron. 2018
Spinal muscular atrophy is an untreatable potentially fatal hereditary disorder caused by loss-of-function mutations in the survival motor neuron (SMN) 1 gene which encodes the SMN protein. Currently, definitive diagnosis relies on the demonstration of biallelic pathogenic variants in SMN1 gene. Therefore, there is an urgent unmet need to accurately quantify SMN protein levels for screening and therapeutic monitoring of symptomatic newborn and SMA patients, respectively. Here, researchers developed a voltammetric immunosensor for the sensitive detection of SMN protein based on covalently functionalized carbon nanofiber-modified screen printed electrodes. A comparative study to Electrochemical detection measurements of rhSMN1 of six different carbon nanomaterial-modified electrodes (carbon, graphene (G), graphene oxide (GO), single wall carbon nanotube (SWCNT), multi-wall carbon nanotube (MWCNT), and carbon nanofiber (CNF)) was performed. The electrochemical characterization and analytical performance of the six immunosensors suggest that carbon nanofiber is a better electrode material for the SMN immunosensor. The voltammetric SMN carbon nanofiber-based immunosensor showed high sensitivity (detection limit of 0.75pg/ml) and selectivity against other proteins such as cystic fibrosis transmembrane conductance regulator (CFTR) and dystrophin (DMD). We suggest that this novel biosensor is superior to other developed assays for SMN detection in terms of lower cost, higher sensitivity, simplicity and capability of high throughput screening.
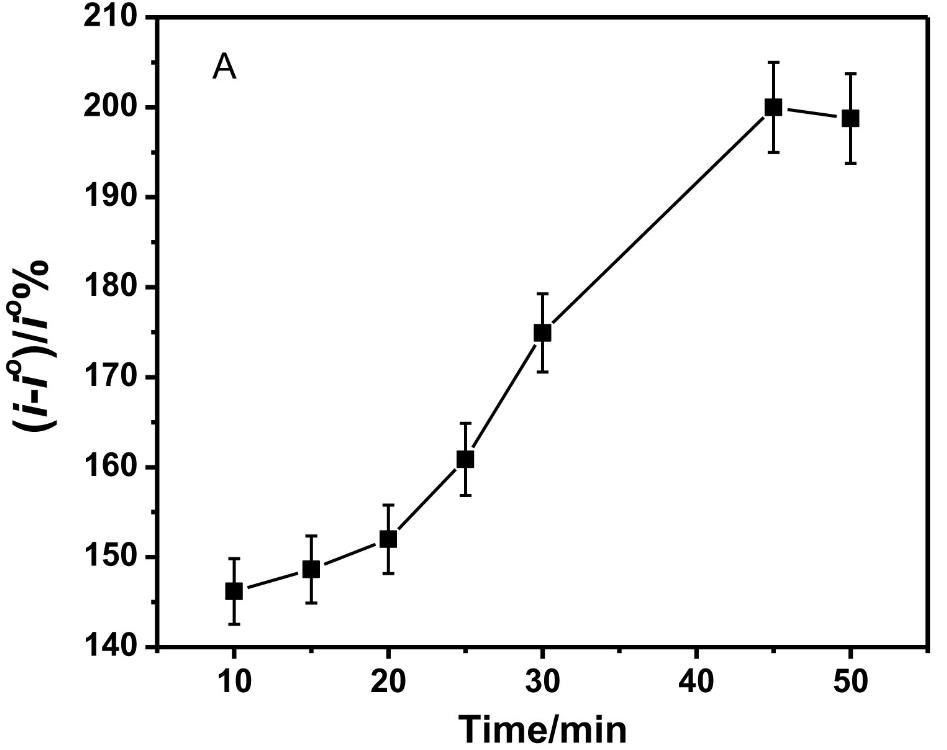
Fig1. CNF immunosensor response with increasing incubation time of the SMN protein.
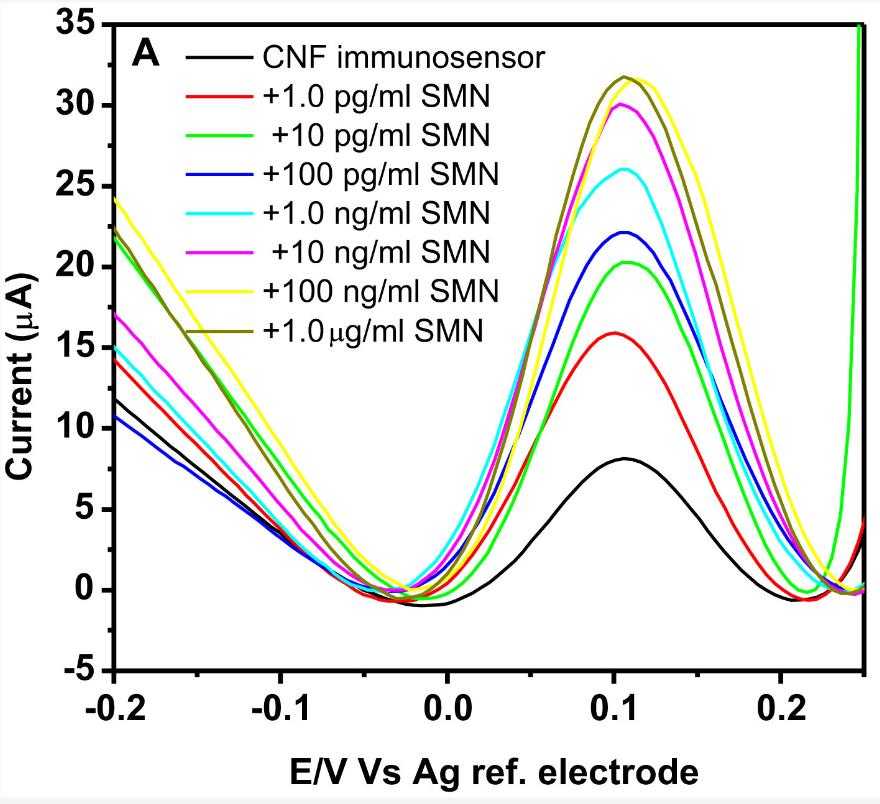
Fig2. SWV of the CNF immunosensor incubated with different concentrations of SMN.
Case 2: Eissa S, et al. Mater Sci Eng C Mater Biol Appl. 2019
The integration of carbon nanomaterials into electrochemical aptasensors has gained significant interest in the recent years because of their high electrical conductivity, mechanical strength, and large surface area. However, no comparative study has been reported so far between different carbon nanomaterials for aptasensing applications. Here, researchers report, a comparative investigation of six carbon electrode materials (carbon, graphene (G), graphene oxide (GO), multi-wall carbon nanotube (MWCNT), single walled carbon nanotube (SWCNT) and carbon nanofiber (CNF)) on the performance of glycated haemoglobin (HbA1c) aptasensor prepared by physical adsorption.
The aptamer adsorption, sensors responses and selectivity of the different nanomaterials including rhSMN protein were compared showing better performance of the SWCNT-based sensor. The voltammetric SWCNT aptasensors showed high sensitivity and selectivity with detection limits of 0.13 pg/mL and 0.03 pg/mL for total haemoglobin (tHb) and HbA1c, respectively. The aptasensor showed selectivity against other proteins in the blood including cystic fibrosis transmembrane conductance regulator (CFTR), survival motor neuron (SMN), dedicator of cytokinesis 8 (DOCK8), signal transducer and activator of transcription 3 (STAT3). This SWCNT aptasensor was superior to the reported detection assays for HbA1c in terms of sensitivity, selectivity and cost.
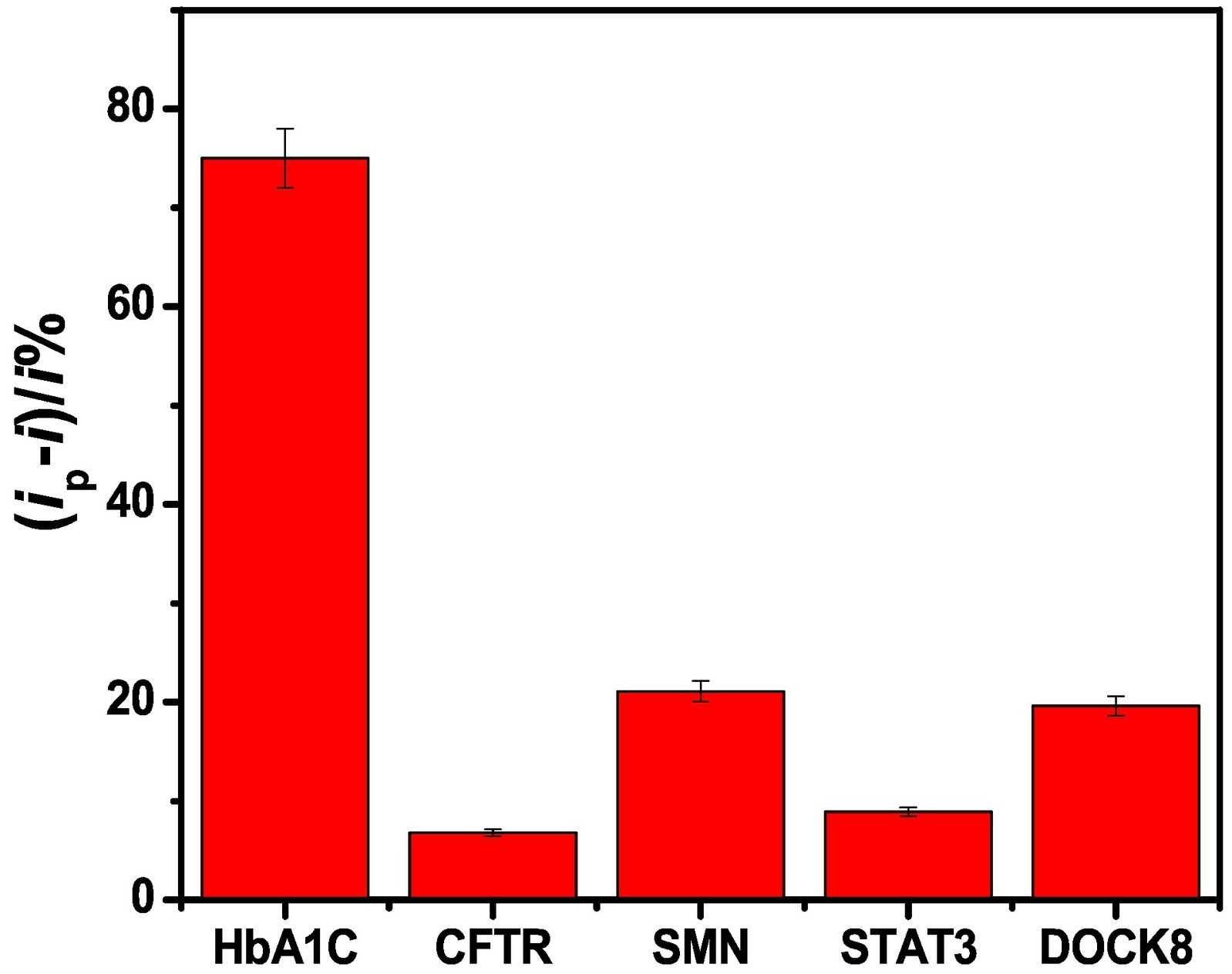
Fig1. The HbA1c aptamer assay selectivity against 1 ng/mL of HbA1c, CFTR, SMN, STAT3 and DOCK8.
Recombinant human SMN1 (rhSMN1) has various potential applications in research and therapeutic settings, particularly in the study and treatment of spinal muscular atrophy (SMA), a genetic disorder caused by mutations in the SMN1 gene. Some potential applications of rhSMN1 include:
SMA Research: rhSMN1 can be used in research studies to better understand the molecular mechanisms underlying SMA and the role of SMN1 in the development and function of motor neurons. This can aid in the development of novel therapeutic strategies for SMA.
Drug Screening: rhSMN1 can be utilized in high-throughput drug screening assays to identify potential small molecules or compounds that can modulate SMN1 expression or function. This can help in the discovery of new drug candidates for the treatment of SMA.
Therapeutic Development: rhSMN1 can serve as a potential therapeutic agent for the treatment of SMA. It can be delivered to patients to restore SMN1 levels and improve motor neuron function, thereby slowing disease progression and improving motor function in SMA patients.
Gene Therapy: rhSMN1 can be used in gene therapy approaches for SMA, where the gene encoding SMN1 is delivered to patients to restore functional SMN expression. This approach can potentially provide a long-term treatment option for SMA patients.
Biomarker Development: rhSMN1 can be used to develop biomarkers for monitoring disease progression, assessing treatment efficacy, and predicting patient outcomes in SMA. This can help in personalized treatment approaches for SMA patients.
Overall, rhSMN1 holds promise for advancing research and therapeutic strategies for SMA, a debilitating genetic disorder with limited treatment options.
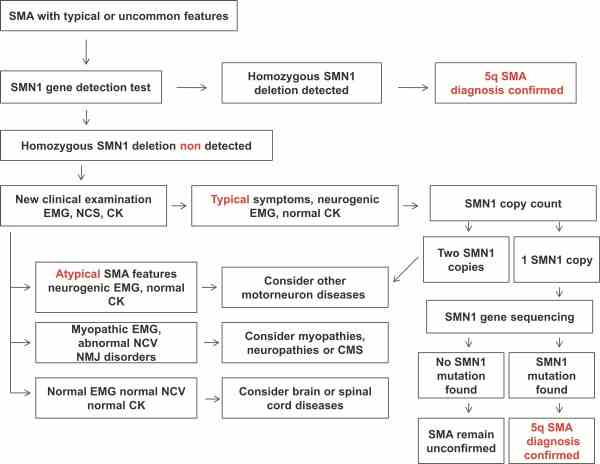
Fig1. Diagnostic algorithm for spinal muscular atrophy. (Adele D'Amico, 2011)
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All SMN1 Products
Required fields are marked with *
My Review for All SMN1 Products
Required fields are marked with *



