Angiogenesis in Cancer
Creative BioMart Angiogenesis in Cancer Product List
Immunology Background
Angiogenesis is a small molecule protein originally isolated from human adenocarcinoma cultured cells. It allows new blood vessels to grow in living tissue. Healthy non-cancerous tissues also produce this small molecule protein, with 35% of the sequences being homologous to pancreatic ribonuclease. Angiogenesis refers to the development of new blood vessels from existing capillaries or posterior veins of capillaries, including: degradation of vascular basement membrane during activation; activation, proliferation, and migration of vascular endothelial cells; reconstruction of new blood vessels and the vascular network is a complex process involving multiple molecules of multiple cells. Angiogenesis is a complex process in which the angiogenic factors and inhibitors coordinate. Under normal conditions, the two are in equilibrium. Once this balance is broken, the vascular system is activated, causing excessive angiogenesis or inhibiting the vascular system to degenerate blood vessels.
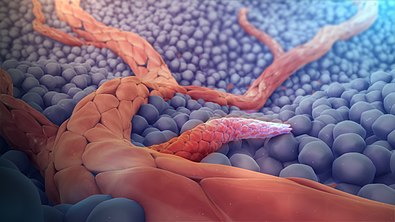 Figure 1. 3D medical animation showing angiogenesis.
Figure 1. 3D medical animation showing angiogenesis.
Introduction
Angiogenesis is the growth of new capillary blood vessels that originate from existing capillaries and post-capillary venules. Tumor angiogenesis is an extremely complex process, which generally includes steps including vascular endothelial matrix degradation, endothelial cell migration, endothelial cell proliferation, endothelial cell tube branching to form a vascular ring, and formation of a new basement membrane. Due to the abnormal structure and function of the neovascularization of the tumor tissue, and the vascular matrix is imperfect, the microvascular is prone to leakage, so the tumor cells do not need to undergo a complicated invasion process and directly penetrate into the blood vessel to enter the bloodstream and are distant. The site forms a metastasis. More and more studies have shown that benign tumors have rare angiogenesis and slow blood vessel growth; most malignant tumors have dense angiogenesis and rapid growth. Therefore, angiogenesis plays an important role in the development and metastasis of tumors. The inhibition of this process will obviously prevent the development and spread of tumor tissues.
Types
1. Tumor angiogenesis
Tumor angiogenesis is an extremely complex process, which generally includes steps including vascular endothelial matrix degradation, endothelial cell migration, endothelial cell proliferation, endothelial cell tube branching to form a vascular ring, and formation of a new basement membrane. The occurrence of tumor angiogenesis is due to the release of angiogenic factors from tumor cells to activate vascular endothelial cells, promote the proliferation and migration of endothelial cells, and on the other hand, the secretion of certain angiogenic factors by endothelial cells stimulates the growth of tumor cells. The interaction between tumor cells and endothelial cells runs through the entire process of tumor angiogenesis. Generally, tumor neocapsules are formed by extending and expanding on the basis of the original blood vessels, and the process is similar to typical wound healing and embryogenesis processes. These new blood vessels provide nutrients to the primary tumor that is continuously infiltrating and growing. In turn, the tumor cells secrete various substances during the growth process to accelerate the formation of new capillary blood vessels.
2. Sprouting angiogenesis
Sprouting angiogenesis is the first form of angiogenesis found. It happens in several well-defined stages. When sprouts extend to angiogenic stimuli, endothelial cells migrate in tandem using adhesion molecules called integrins. As the cells migrate to the angiogenic site, these sprouts then form a loop that becomes a complete vascular lumen. Germination occurs at a rate of a few millimeters per day and allows new blood vessels to grow in the gaps of the vasculature. It is significantly different from mitotic angiogenesis because it forms a new blood vessel rather than splitting existing blood vessels.
3. Intussusception angiogenesis
In this type of blood vessel formation, the capillary wall extends into the lumen to divide a single blood vessel into two parts. Intussusception angiogenesis has four stages. First, two opposing capillary walls establish a contact area. Second, the endothelial cell junction is recombined and the vascular bilayer is perforated to allow growth factors and cells to penetrate into the lumen. Third, a nucleus is formed in the contact area between the two new blood vessels, which is filled with pericytes and myofibroblasts. These cells begin to lay collagen fibers into the core to provide an extracellular matrix for the growth of the lumen of the blood vessel. In the end, the core was enriched and the basic structure did not change. Intussusception is important because it is a reorganization of existing cells. It allows a large increase in the number of capillaries and a corresponding increase in the number of endothelial cells. This is especially important in embryonic development because there are not enough resources to create a rich microvasculature with new cells each time a new blood vessel is developed.
Chemical stimulation
Chemical stimulation of angiogenesis is performed by various angiogenic proteins e.g integrins and prostaglandins, including several growth factors e.g. VEGF, FGF.
1. Vascular endothelial growth factor (VEGF)
Vascular endothelial growth factor (VEGF), as shown in Figure 2, which is originally known as vascular permeability factor (VPF), is a signaling protein that stimulates angiogenesis in cells. In particular, VEGF is a subfamily of growth factors and is a family of growth factors of the platelet-derived cystine growth factor family. They are important signaling proteins involved in angiogenesis (former formation of the embryonic circulatory system) and angiogenesis (vascular growth from existing blood vessels). It is part of the system and can restore the oxygen supply to the tissue when blood circulation is insufficient (for example, under hypoxic conditions). The concentration of VEGF in the serum of patients with bronchial asthma and diabetes is higher. When VEGF is overexpressed, it may cause disease. Without adequate blood supply, solid cancer cannot develop to a limited scale. Cancers that express VEGF are capable of growth and metastasis. Overexpression of VEGF can cause retinal vascular disease in the eye and other parts of the body.
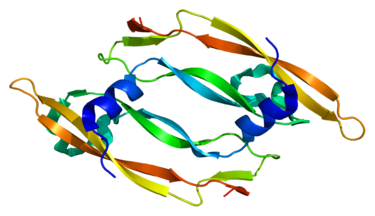 Figure 2. Structure of VEGF.
Figure 2. Structure of VEGF.
2. Fibroblast growth factor (FGF)
Fibroblast growth factor (FGF) is a class of cellular signal transduction proteins involved in a variety of processes, most notably as a key element of normal development. Any irregularity in their function can lead to a series of developmental defects. These growth factors are commonly used as systems or local circulating molecules that activate the extracellular origin of cell surface receptors. The main feature of FGFs is that they bind to heparin and heparan sulfate, and it is therefore found that some of them are sequestered in the extracellular matrix of tissues containing heparan sulfate proteoglycans and are locally released upon injury or tissue remodeling.
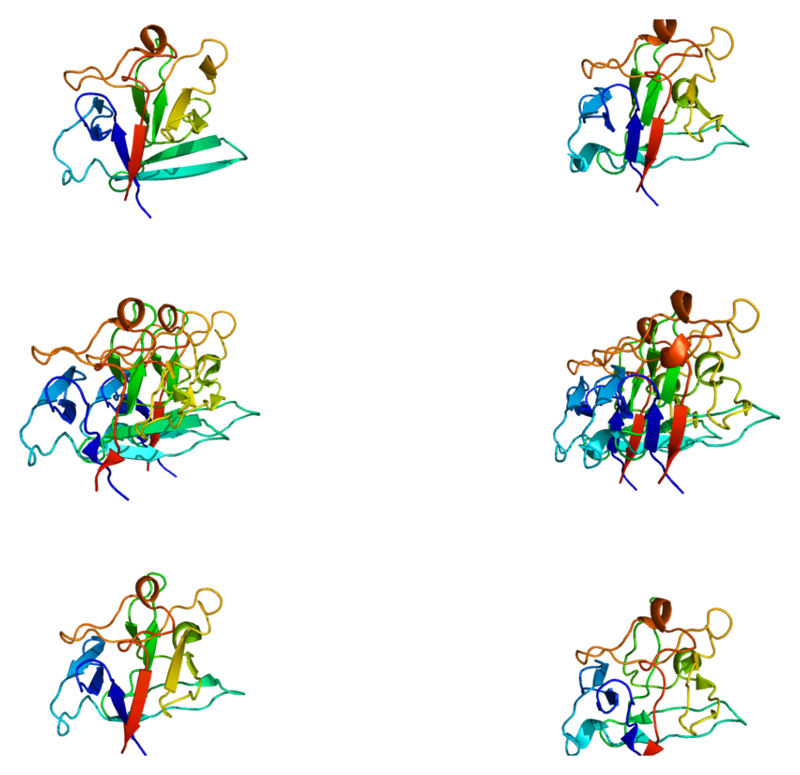 Figure 3. Structure of the FGF1 protein.
Figure 3. Structure of the FGF1 protein.
References:
1. Liotta LA.; et al. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980.284 (5751): 67–8.
2. Wang JH.; et al. Mechanoregulation of gene expression in fibroblasts. Gene. 2007,391 (1–2): 1–15.

