H2A Family
Related Symbol Search List
- H2AFB1
- H2AFB2
- H2AFB3
- H2AFJ
- H2AFV
- H2AFX
- H2AFY
- H2AFZ
- HIST1H2AA
- HIST1H2AB
- HIST1H2AC
- HIST1H2AD
- HIST1H2AE
- HIST1H2AG
- HIST1H2AI
- HIST1H2AJ
- HIST1H2AK
- HIST1H2AL
- HIST1H2AM
- HIST2H2AA3
- HIST2H2AC
Immunology Background
Background
Histones H2A are essential components of nucleosomes, which are the basic units of chromatin structure. Histone H2A proteins are composed of ∼130 amino acid residues. The H2A family is one of the most sequence-divergent families, including macroH2A, H2A. X, H2A. Z and H2A-Bbd. The structure of histones H2A plays a crucial role in epigenetic regulation of gene expression, DNA repair, and chromatin organization. Here are the details on the structure of histones H2A and their functions:
Structure of Histones H2A
Histones H2A have a highly conserved structure consisting of a central globular domain and a flexible N-terminal tail. The globular domain interacts with other histones, including H2B, H3, and H4, to form the core of the nucleosome. The N-terminal tail extends outward from the nucleosome and can undergo various post-translational modifications, such as acetylation, methylation, phosphorylation, and ubiquitination. These modifications contribute to the regulation of chromatin structure and gene expression.
Functions in Epigenetic Regulation of Gene Expression
- Chromatin Packaging: Histones H2A, along with other core histones, help package DNA into a compact structure called chromatin. This packaging influences the accessibility of DNA to transcription factors and other regulatory proteins. Modifications of H2A, such as acetylation or methylation of its N-terminal tail, can alter chromatin structure, impacting gene expression by promoting or inhibiting transcription.
- Transcriptional Regulation: The modifications on the N-terminal tail of H2A, known as histone marks, can serve as binding sites for various protein complexes involved in gene regulation. For example, acetylation of H2A is associated with transcriptional activation, whereas methylation at specific residues can be linked to gene repression or activation, depending on the context. These modifications influence the recruitment of transcriptional machinery and chromatin remodelers, thereby regulating gene expression.
Functions in DNA Repair
- DNA Damage Recognition: Histone H2A.X is specifically involved in the DNA damage response. When DNA double-strand breaks occur, H2A.X gets rapidly phosphorylated at the site of the break, creating a signal that attracts DNA repair proteins to facilitate repair processes.
- DNA Repair Pathways: H2A.X plays a critical role in DNA repair pathways, such as homologous recombination and non-homologous end joining. It helps recruit repair factors and coordinate the repair process, ensuring the accurate and timely repair of DNA lesions.
Functions in Chromatin Organization
- Nucleosome Stability: Histones H2A contribute to the stability and integrity of nucleosomes. They interact with DNA and other histones, maintaining the proper spacing between nucleosomes and compacting DNA into higher-order chromatin structures.
- Chromatin Remodeling: H2A variants, such as H2A.Z, play a role in chromatin remodeling by altering nucleosome positioning and stability. H2A.Z is involved in the regulation of gene expression, facilitating access to DNA by transcription factors or other regulatory proteins.
- Higher-Order Chromatin Structure: Histones H2A, along with other core histones, contribute to the formation of higher-order chromatin structures, such as chromatin loops and domains. These structures help organize the genome and regulate the interactions between regulatory elements and target genes.
In summary, histones H2A have a fundamental role in epigenetic regulation, DNA repair, and chromatin organization. Their structure and modifications contribute to the regulation of gene expression, maintenance of genome integrity through DNA repair, and the establishment of chromatin structure at various levels.
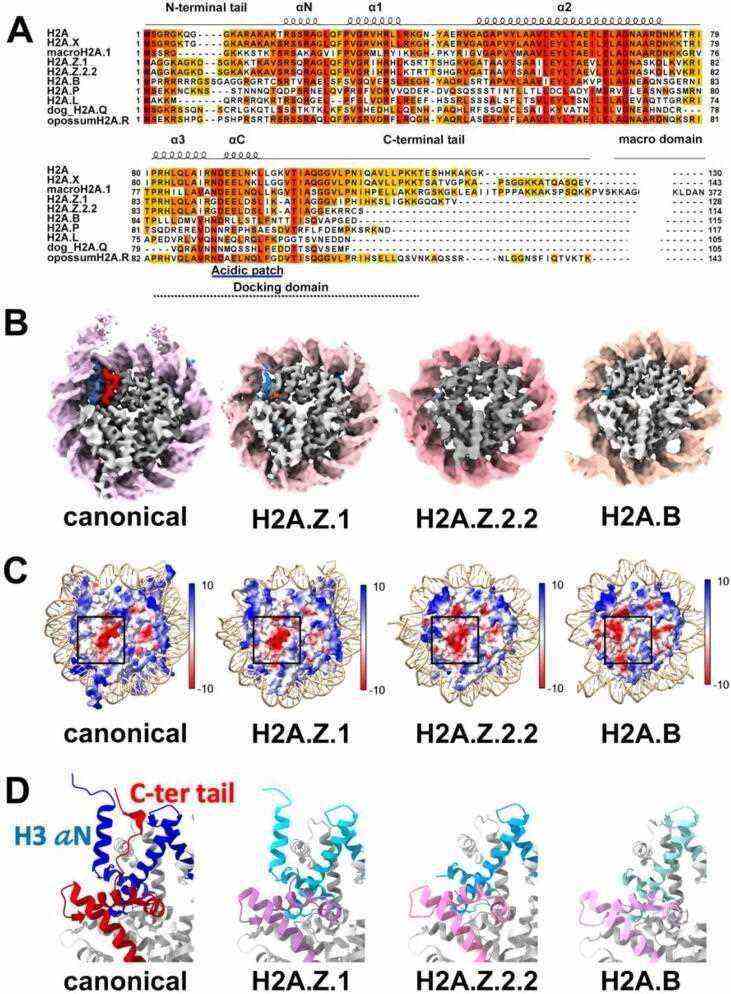 Fig.1 Structural diversity of histone H2A variants, H2A.Z and H2A.B. (Sokolova V, et al., 2022)
Fig.1 Structural diversity of histone H2A variants, H2A.Z and H2A.B. (Sokolova V, et al., 2022)Members and Variants of the H2A Family
The H2A family consists of several members, each with distinct properties and functions. Here are some prominent members of the H2A family:
| Members and variants | Details |
|---|---|
| H2A.1 | H2A.1, also known as H2A.1H and HIST1H2AA, is the canonical form of H2A and is widely expressed in most cell types. Its primary function is in general chromatin packaging and gene regulation. As a component of nucleosomes, H2A.1 helps package DNA into a compact structure called chromatin, contributing to the organization and compaction of the genome. It plays a role in regulating gene expression by influencing the accessibility of DNA to transcription factors and other regulatory proteins. |
| H2A.Z | H2A.Z is a highly conserved histone variant that plays a crucial role in gene expression regulation, often found near gene promoters and associated with both transcriptional activation and repression. It is involved in various cellular processes, including embryonic development, differentiation, and response to environmental signals. H2A.Z.1 and H2A.Z.2 are two subtypes of H2A.Z with similar functions in transcriptional regulation and chromatin remodeling.
|
| H2A.X | H2A.X (H2AFX) is a variant of H2A that is specifically involved in DNA damage response and repair processes. It is rapidly phosphorylated at sites of DNA double-strand breaks and recruits repair factors to facilitate DNA repair and maintain genome stability. |
| MacroH2A | MacroH2A is a variant of histone H2A that contains a macrodomain and is associated with gene silencing and heterochromatin formation.
|
| H2A.Bbd | 2A.Bbd (Barr body-deficient), also known as H2A.B, is a variant of H2A found predominantly in mammalian testes. It is involved in the replacement of canonical H2A in sperm and plays a role in chromatin remodeling during spermatogenesis. |
These are just a few examples of H2A family members, and there are other variants or isoforms, such as H2A.J (H2AFJ), H2A.V (H2AFV), H2A.L (H2AFL), H2A.B (H2AFB), H2A.W (H2AFW), H2A.O (H2AFO), H2A.P (H2AFP), H2A.L.2 (H2AFL2), H2A.Q (H2AFQ), H2A.Y (H2AFY), H2A.P.2 (H2AFZP2). The functions of these variants vary, including roles in chromatin packaging, gene regulation, transcriptional activation or repression, DNA damage response and repair, spermatogenesis, and heterochromatin formation. The diversity of H2A variants increases the complexity of chromatin structure and its dynamic regulation in various biological processes.
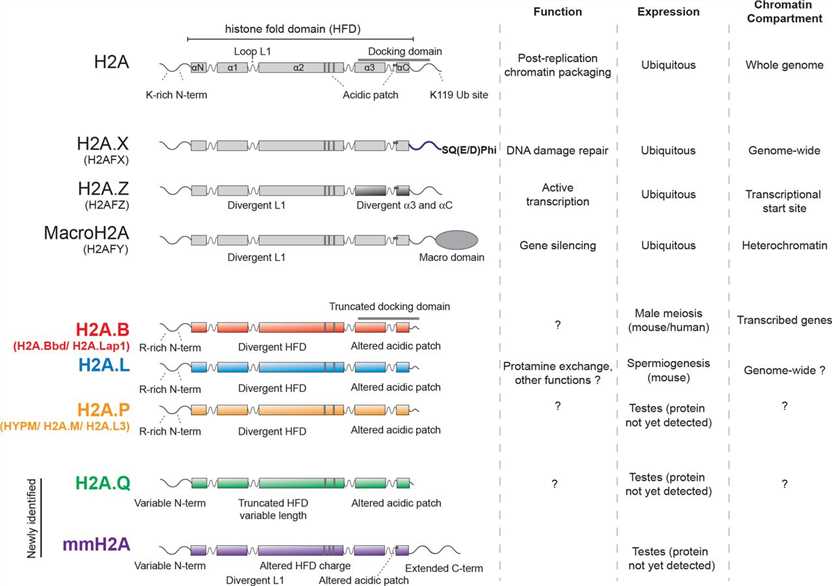 Fig.2 Features of H2A protein families. (Molaro A, et al., 2018)
Fig.2 Features of H2A protein families. (Molaro A, et al., 2018)Role of Histone H2A Family in Various Diseases
Dysregulated expression of histones, including H2A variants, has been implicated in various diseases, including cancer, neurodegenerative disorders, and developmental abnormalities. Here's a discussion on the implications of dysregulated histone H2A expression in these diseases:
Cancer
Dysregulation of histone H2A expression has been observed in different types of cancers. Alterations in H2A expression levels or mutations in H2A genes can disrupt normal chromatin structure and gene regulation, leading to abnormal cell growth, proliferation, and the development of tumors. For example, changes in the expression of H2A.Z variants have been associated with several types of cancer, including breast, prostate, and colorectal cancer. Dysregulated H2A expression can influence gene expression patterns, contribute to genomic instability, and affect the response to DNA damage, thereby promoting tumor development and progression.
Neurodegenerative Disorders
Dysregulation of histone H2A expression has also been linked to neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, and Huntington's disease. In these conditions, aberrant histone modifications and altered chromatin structure can impact gene expression patterns, leading to the dysfunction and degeneration of neurons. H2A variants, including H2A.Z and H2A.X, have been implicated in neurodegenerative processes, including DNA damage repair, oxidative stress response, and the regulation of genes associated with neuronal function and survival.
Developmental Abnormalities
Dysregulated expression of H2A variants can contribute to developmental abnormalities and birth defects. During embryonic development, precise regulation of gene expression is critical for proper cell differentiation and organ formation. Alterations in histone H2A expression or modifications can disrupt the normal epigenetic landscape, leading to developmental defects. For instance, abnormal expression of H2A.Z has been associated with developmental abnormalities in zebrafish and mouse models, indicating its importance in embryonic development and organogenesis.
Understanding the specific roles of H2A variants and their dysregulation in these diseases can provide valuable insights into disease mechanisms and potential therapeutic targets. Further research is needed to elucidate the precise mechanisms by which dysregulated H2A expression contributes to these diseases and to explore the therapeutic potential of targeting histone modifications and chromatin remodeling in disease treatment and prevention.
Case Study
Case 1: Wu XM, Fang H, Zhang J, Bi YH, Chang MX. Histone H2A nuclear/cytoplasmic trafficking is essential for negative regulation of antiviral immune response and lysosomal degradation of TBK1 and IRF3. Front Immunol. 2021;12:771277.
The study focused on the expression and localization of zebrafish histone H2A (GenBank accession number MK800012) in response to bacterial and viral infections. Zebrafish larvae were infected with SVCV, and the expression of SVCV-N gene and histone H2A was analyzed at different time points. The expression of SVCV-N gene increased significantly over time, while histone H2A showed a down-regulation at 6 and 12 hours post-infection (hpi) and an up-regulation at 48 and 72 hpi.
Confocal microscopy analysis revealed that histone H2A was initially localized only in the nucleus of infected cells at 6 hpi, but at later time points (12, 24, and 48 hpi), it was found in both the cytoplasm and nucleus of infected cells.
Subcellular fractionation experiments showed that the nuclear/cytoplasmic trafficking of histone H2A was observed only at higher multiplicities of infection (MOIs) of SVCV (MOI 1, 5, or 10), but not at a lower MOI (0.1).
Further experiments using ZF4 and EPC cells confirmed the nuclear/cytoplasmic trafficking of histone H2A in response to SVCV infection and poly I:C stimulation. The protein expression of histone H2A in the cytoplasm was induced by both poly I:C and SVCV infection, while the nuclear expression of histone H2A was decreased by SVCV infection.
In summary, the study demonstrated that SVCV infection and poly I:C stimulation led to the nuclear/cytoplasmic trafficking of histone H2A in zebrafish and EPC cells, indicating the dynamic regulation of histone localization in response to different stressors.
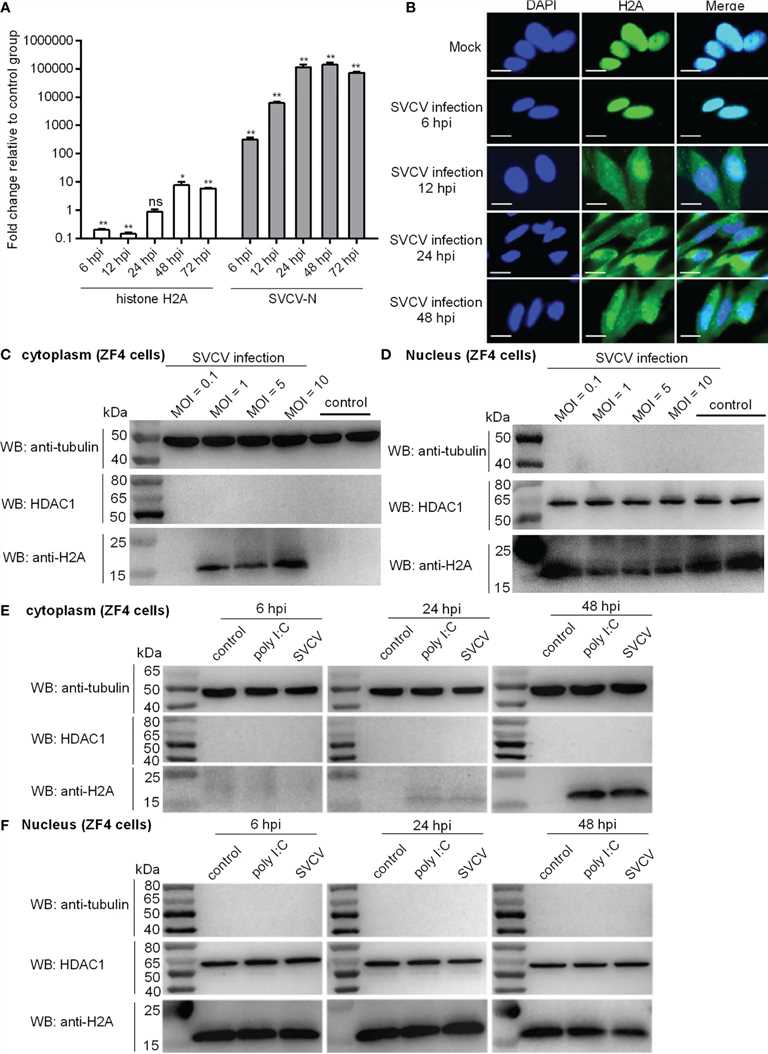 Fig.1 Expression and subcellular localization of zebrafish histone H2A.
Fig.1 Expression and subcellular localization of zebrafish histone H2A.Case 2: Wu BJ, Dong FL, Ma XS, Wang XG, Lin F, Liu HL. Localization and expression of histone H2A variants during mouse oogenesis and preimplantation embryo development. Genet Mol Res. 2014;13(3):5929-5939.
The study investigated the localization of histone H2A variants during mouse oogenesis. Immunocytochemistry was performed on oocytes at different stages of growth using specific antibodies for various histone H2A variants.
The results showed that H2A.Bbd signal was absent from 1-, 10-, and 15-day oocyte chromatin, indicating its localization in the cytoplasm. However, in 5-day oocytes, H2A.Bbd was deposited around the nuclear area, suggesting its assembly from the cytoplasm to the nuclear region. In 20-day oocytes, H2A.Bbd signal was detected in both the nucleus and cytoplasm.
Similarly, H2A.Z signal was absent from 1-, 5-, 10-, and 15-day oocyte chromatin, indicating its cytoplasmic localization during early oocyte growth. However, in 20-day oocytes, H2A.Z signal started to be deposited in the nucleus.
On the other hand, abundant H2A.X signal was present in 1-, 10-, 15-, and 20-day oocyte chromatin, except for 5-day oocytes where it was localized around the nuclear area.
Overall, the study revealed distinct localization patterns for histone H2A variants during mouse oogenesis. H2A.Bbd showed cytoplasmic localization with nuclear accumulation in 5-day oocytes, H2A.Z exhibited cytoplasmic localization with nuclear deposition in 20-day oocytes, and H2A.X was predominantly localized in the growing oocyte chromatin, except for 5-day oocytes where it was around the nuclear area.
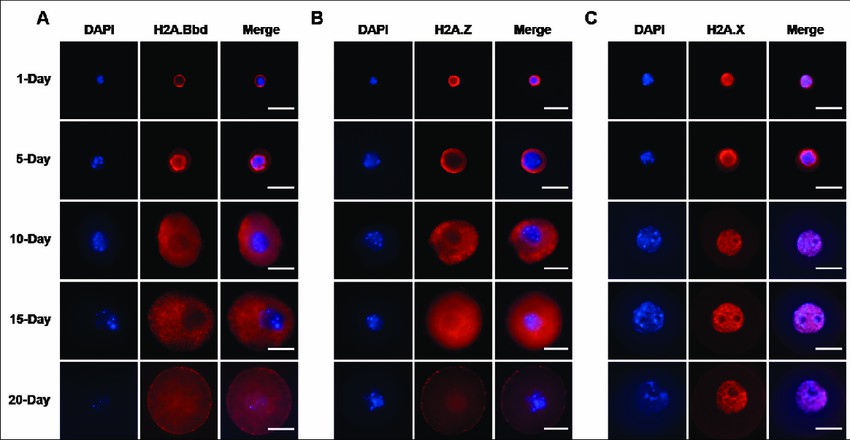 Fig.2 Localization and expression of histone H2A variants during mouse oogenesis.
Fig.2 Localization and expression of histone H2A variants during mouse oogenesis.References
- Molaro A, Young JM, Malik HS. Evolutionary origins and diversification of testis-specific short histone H2A variants in mammals. Genome Res. 2018;28(4):460-473.
- Sokolova V, Sarkar S, Tan D. Histone variants and chromatin structure, update of advances. Comput Struct Biotechnol J. 2022;21:299-311.

