Neural Stem Cells
Creative BioMart Neural Stem Cells Product List
Immunology Background
Background
Neural stem cells (NSCs) are a type of stem cell found in the central nervous system (CNS) that has the ability to self-renew and differentiate into various types of neurons and glial cells. They are essential for neurogenesis, the process of generating new neurons, particularly during development and in response to injury.
Characteristics of Neural Stem Cells
|
1. Self-renewal NSCs can divide to produce more stem cells, maintaining their population over time. |
|
2. Pluripotency NSCs are multipotent, meaning they can differentiate into various cell types within the nervous system, including:
|
|
3. Location NSCs are primarily located in specific regions of the brain, such as:
|
|
4. Response to Signals NSCs respond to various intrinsic and extrinsic signals, such as growth factors and environmental cues, which influence their maintenance, migration, and differentiation. |
|
5. Plasticity NSCs exhibit a degree of plasticity, enabling them to adapt to different roles in neural repair and regeneration. |
|
6. Quiescence NSCs can enter a quiescent state, reducing their metabolic activity and proliferation, which allows them to preserve their function over time. |
|
7. Role in Development and Repair In addition to contributing to normal brain development, NSCs are crucial for repairing damaged tissue in the CNS, offering the potential for therapies in neurodegenerative diseases and injuries. |
Neural stem cells play a vital role in the development and maintenance of the central nervous system, with unique properties that make them valuable for research and potential therapeutic interventions. Understanding their characteristics helps pave the way for advances in neurobiology, regenerative medicine, and treatments for neurological disorders.
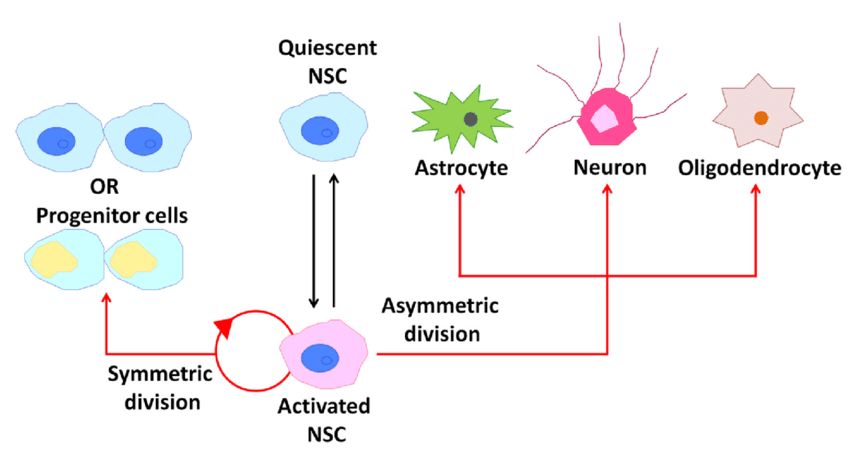 Fig.1 Behavior of neural stem cells (NSCs) within the adult mammalian brain. (Takahashi T, 2021)
Fig.1 Behavior of neural stem cells (NSCs) within the adult mammalian brain. (Takahashi T, 2021)Functions and Roles of Neural Stem Cells
NSCs play crucial roles in the development and maintenance of the nervous system. Here are their key functions and roles:
Neurogenesis: NSCs are responsible for generating new neurons during development and throughout life, particularly in regions like the hippocampus.
Astrocyte and Oligodendrocyte Formation: In addition to neurons, NSCs can differentiate into glial cells such as astrocytes and oligodendrocytes, which are essential for supporting neuronal function and myelination.
Regeneration and Repair: NSCs have the potential to aid in the repair of damaged neural tissues after injury or disease, contributing to the regeneration of neurons and supporting cells.
Homeostasis Maintenance: NSCs help maintain the balance of neural cell types and the overall integrity of the neural environment, responding to physiological changes and stress.
Role in Neurodegenerative Diseases: NSCs may be involved in the pathophysiology of neurodegenerative diseases and hold potential for therapeutic applications, offering avenues for cell replacement and repair strategies.
Inflammation Regulation: NSCs can influence inflammation within the nervous system, contributing to the modulation of immune responses in neuroinflammatory conditions.
Plasticity and Cognitive Function: NSCs play a role in synaptic plasticity and cognitive functions, impacting learning and memory by generating new neurons that integrate into existing circuits.
Overall, NSCs are vital for both the development and ongoing functionality of the central nervous system, with significant implications for understanding and treating neurological disorders.
Key Components of Neural Stem Cells
NSCs are characterized by specific markers, growth factors, and transcription factors that play essential roles in their maintenance, differentiation, and regulation. Here is an introduction to these key components:
Neural Stem Cell Differentiation Markers
Neural stem cell differentiation markers are proteins or molecules that indicate the progression of NSCs towards specific neural cell types. These markers help identify the stage of differentiation and the type of neural cells being produced. Some common NSC differentiation markers include:
- Nestin: Nestin is a type VI intermediate filament protein commonly expressed in neural stem/progenitor cells during development and in adult neurogenic regions.
- β-III Tubulin (Tuj1): This protein is a neuronal marker expressed during neuronal differentiation and is used to identify immature neurons derived from NSCs.
- Glial Fibrillary Acidic Protein (GFAP): GFAP is a marker for astrocytes, a type of glial cell, and is expressed during astrocytic differentiation of NSCs.
Neural Stem Cell Growth Factors
Neural stem cell growth factors are proteins that regulate the proliferation, survival, and differentiation of NSCs. These factors play crucial roles in maintaining the stem cell population and promoting their differentiation into neural cell types. Some important NSC growth factors include:
- Epidermal Growth Factor (EGF): EGF is a mitogen that stimulates the proliferation of NSCs and helps maintain their stemness.
- Basic Fibroblast Growth Factor (bFGF or FGF-2): bFGF promotes the self-renewal of NSCs and supports their survival and proliferation.
- Brain-Derived Neurotrophic Factor (BDNF): BDNF is a neurotrophic factor that promotes the survival and differentiation of neurons derived from NSCs.
Neural Stem Cell Markers
Neural stem cell markers are proteins or molecules expressed specifically in NSCs, helping to identify and isolate these cells. These markers are often used in research and therapeutic applications involving NSCs. Some common NSC markers include:
- Sox2: Sox2 is a transcription factor critical for maintaining NSC self-renewal and pluripotency.
- Pax6: Pax6 is a transcription factor involved in NSC proliferation and differentiation, particularly in the development of the nervous system.
- CD133 (Prominin-1): CD133 is a surface marker commonly used to isolate and characterize neural stem and progenitor cells.
Neural Stem Cell Transcription Factors
Neural stem cell transcription factors are proteins that regulate gene expression and control the fate of NSCs during development and in adult neurogenic niches. These factors orchestrate the molecular processes underlying NSC proliferation, differentiation, and maintenance. Some key NSC transcription factors include:
- Sox2: As mentioned earlier, Sox2 is a crucial transcription factor for maintaining NSC pluripotency and self-renewal.
- Nestin: Nestin not only serves as a differentiation marker but also functions as a transcription factor regulating the expression of genes involved in NSC development.
- Olig2: Olig2 is a transcription factor important for the specification of neural progenitor cells and the generation of oligodendrocytes from NSCs.
These markers, factors, and transcription factors collectively contribute to the understanding and manipulation of neural stem cells for both research and therapeutic purposes.
Potential Roles and Applications of NSCs in Disease Progression and Treatment
NSCs have shown great promise in understanding disease progression and offering therapeutic interventions across a spectrum of neurological disorders. Here are some potential roles and applications of NSCs in disease progression and treatment, along with examples of their utilization:
| Disease | Role in Disease Progression | Therapeutic Application |
|---|---|---|
| Parkinson's Disease | NSCs can differentiate into dopamine-producing neurons that are lost in Parkinson's disease, potentially offering a means to replace damaged neurons. | Transplantation of NSCs has been explored as a potential treatment to replenish dopamine-producing neurons in the brain and alleviate motor symptoms. |
| Alzheimer's Disease | NSCs can potentially differentiate into various neural cell types, offering the possibility of replacing damaged or degenerating neurons in regions affected by Alzheimer's disease. | NSCs could be engineered to produce and release neurotrophic factors that promote neuronal survival and counteract the neurodegenerative processes seen in Alzheimer's disease. |
| Spinal Cord Injury | NSCs have the ability to migrate to sites of spinal cord injury and promote tissue repair and regeneration by differentiating into neural cells. | Transplantation of NSCs into the injured spinal cord aims to replace damaged cells, promote axon regrowth, and improve functional recovery in patients with spinal cord injuries. |
| Stroke | NSCs can migrate to regions of brain injury caused by stroke and differentiate into neurons and glial cells, potentially contributing to tissue repair. | NSC transplantation post-stroke has been investigated as a means to promote brain repair, enhance functional recovery, and reduce post-stroke disabilities. |
| Multiple Sclerosis | NSCs have immunomodulatory properties and can potentially regulate the inflammatory response in multiple sclerosis. | NSCs may be harnessed to modulate the immune response, promote myelin repair, and protect against further demyelination in multiple sclerosis patients. |
| Brain Tumors | NSCs have been explored for their tumor-tropic properties, allowing them to home in on brain tumors. | NSCs can be engineered to deliver therapeutic agents directly to brain tumor sites, offering a targeted and potentially more effective treatment approach. |
In summary, NSCs hold significant potential in understanding disease progression and providing innovative therapeutic strategies across a range of neurological disorders, offering hope for improved treatments and better outcomes for patients
Research Progress of Neural Stem Cells
1. Understanding Neural Stem Cell Behavior
Recent studies have provided deeper insights into the behavior of neural stem cells (NSCs). Researchers have identified key molecular pathways that regulate NSC proliferation, differentiation, and maintenance. For instance, the role of the Notch signaling pathway has been re-evaluated, revealing its critical function in maintaining NSC populations and preventing premature differentiation.
2. Microscale Environment and NSC Niche
New advancements in tissue engineering techniques have allowed scientists to recreate the NSC niche in vitro. These engineered environments mimic the biochemical and mechanical properties of the brain, facilitating more accurate studies of NSC behavior and their interactions with neighboring cells. This helps in understanding how extrinsic factors influence NSC fate and functionality.
3. CRISPR and Gene Editing Technologies
The application of CRISPR-Cas9 technology has made it possible to edit genes in NSCs with unprecedented precision. By knocking out or activating specific genes, researchers can explore their functions in NSC biology and potentially correct genetic mutations that lead to neurological disorders. This technique holds promise for developing targeted therapies for conditions like Alzheimer's and Parkinson's disease.
4. Regenerative Medicine Applications
Advancements in NSC research are paving the way for regenerative medicine applications. Studies have demonstrated that NSCs can be transplanted to repair damaged brain regions, offering hope for treating traumatic brain injuries and neurodegenerative diseases. Clinical trials are underway to assess the safety and efficacy of NSC-based therapies in humans.
5. Exosomal Communication
Recent discoveries have highlighted the role of exosomes—small vesicles released by cells—in NSC communication. Exosomes from NSCs can carry proteins and RNAs that modulate the behavior of adjacent cells, influencing neurogenesis and neuroprotection. This finding opens new avenues for understanding how NSCs interact with their environment and potentially leveraging exosomes for therapeutic applications.
6. Induced Pluripotent Stem Cells (iPSCs)
The ability to generate NSCs from iPSCs has revolutionized the field. Recent breakthroughs have optimized protocols for differentiating iPSCs into NSCs, enabling the generation of patient-specific cells. This holds significant potential for disease modeling, drug screening, and personalized medicine.
7. Role of the Microbiome
Emerging research is exploring the connection between the gut microbiome and NSC activity. Studies suggest that gut-derived metabolites can influence neurogenesis and brain health, leading to potential new therapeutic targets for enhancing NSC function through microbiome modulation.
8. Single-Cell Technologies
The advent of single-cell RNA sequencing has allowed scientists to profile individual NSCs, revealing heterogeneity within NSC populations. This understanding can lead to refined approaches in targeting specific stem cell subtypes for therapy and investigating how different signals affect their fate decisions.
9. Stem Cell Aging and Senescence
Recent breakthroughs have shed light on the mechanisms of NSC aging and senescence, which contribute to age-related cognitive decline. Understanding these processes may aid in developing interventions to rejuvenate NSCs in older individuals, addressing neurological diseases prevalent in the aging population.
10. Optogenetics and Neural Circuitry
Combining NSCs with optogenetics has allowed researchers to manipulate and control neuronal activity with light, providing insights into neural circuitry and behavior. This technology has the potential to uncover new therapeutic strategies for neurological disorders.
11. Bioengineering Approaches
The integration of NSCs with biomaterials and bioengineering techniques has led to the development of innovative strategies for NSC transplantation, tissue engineering, and neural regeneration. These approaches aim to improve the survival, integration, and functionality of transplanted NSCs in the brain.
12. Neuroinflammation and Immunomodulation
Researchers are exploring the role of NSCs in modulating neuroinflammatory responses in various neurological conditions. Understanding how NSCs interact with the immune system and influence inflammation could lead to novel therapeutic approaches for neuroinflammatory disorders.
13. Clinical Trials and Translational Research
Advances in NSC research have paved the way for several ongoing clinical trials investigating the safety and efficacy of NSC-based therapies for neurological disorders such as spinal cord injury, stroke, and neurodegenerative diseases. These trials represent a significant step towards translating NSC research into clinical applications.
Neural stem cell research is rapidly advancing, with new discoveries promising to enhance our understanding of brain development, repair mechanisms, and neurological diseases. Continued exploration in this field holds great potential for therapeutic innovations in regenerative medicine and neuroprotection.
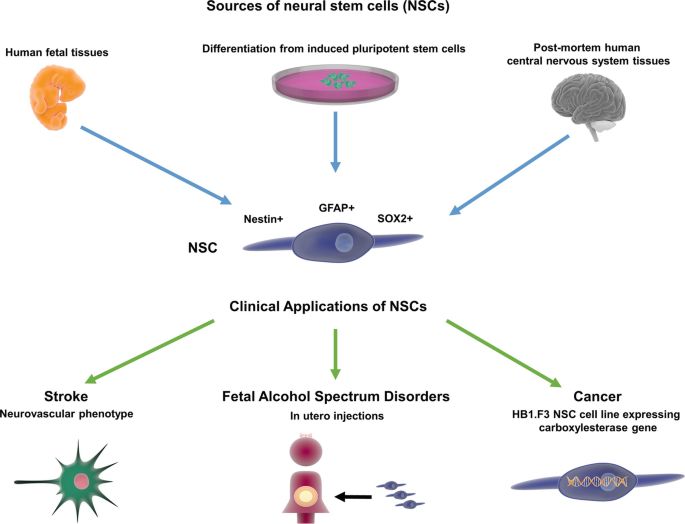 Fig.2 Sources and clinical applications of NSCs. (Tuazon JP, et al., 2019)
Fig.2 Sources and clinical applications of NSCs. (Tuazon JP, et al., 2019)Case Study
Case 1: Hwang DH, Kim BG, Kim EJ, et al. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 2009;10:117.
Contusive spinal cord injuries are complicated by a delayed loss of oligodendrocytes, leading to progressive demyelination. Transplantation strategies providing oligodendrocyte lineage cells to enhance myelination seem promising for spinal cord repair. Researchers investigated whether transplanting human neural stem cells (NSCs) engineered to express the Olig2 transcription factor could improve locomotor recovery and myelination in a rat contusive spinal cord injury model.
The study involved transducing the HB1.F3 (F3) human NSC line with a retroviral vector encoding Olig2, promoting NSC differentiation into oligodendrocyte lineage cells. F3.Olig2 NSCs exhibited increased proliferation and migration towards white matter, resulting in enhanced myelination and improved hindlimb locomotion in animals receiving these grafts. The findings suggest that manipulating molecular factors like Olig2 could enhance the reparative potential of cell-based therapies for spinal cord trauma.
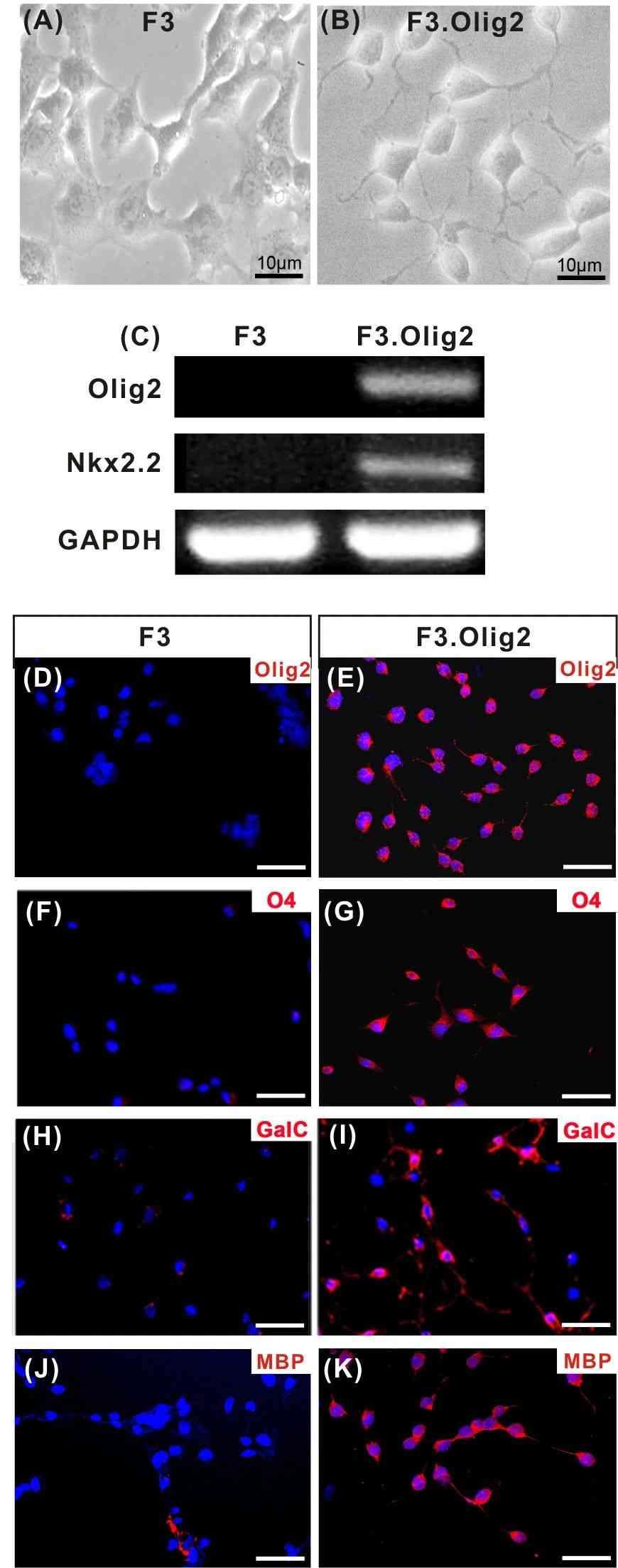 Fig.1 Characterization of human neural stem cells (NSCs) transduced with Olig2 transcription factor.
Fig.1 Characterization of human neural stem cells (NSCs) transduced with Olig2 transcription factor.Case 2: Zhang R, Mao W, Niu L, et al. NSC-derived exosomes enhance the therapeutic effects of NSC transplantation on cerebral ischemia in mice. Elife. 2023;12:e84493.
The transplantation of neural stem cells (NSCs) has been demonstrated to enhance the functional recovery of brain lesions, such as ischemic stroke. However, the efficacy of NSC transplantation is hampered by the challenges of low cell survival and differentiation rates within the hostile post-stroke brain environment. In a recent study, researchers utilized NSCs derived from human induced pluripotent stem cells in conjunction with exosomes sourced from NSCs to address cerebral ischemia resulting from middle cerebral artery occlusion/reperfusion in mice. The findings revealed that NSC-derived exosomes notably dampened the inflammatory response, mitigated oxidative stress post-NSC transplantation, and promoted NSC differentiation in vivo. The combined therapy of NSCs with exosomes mitigated brain tissue damage, including cerebral infarction, neuronal loss, and glial scarring, while fostering the restoration of motor function. Through an analysis of the miRNA profiles of NSC-derived exosomes and their potential downstream gene targets, the study shed light on the mechanisms underpinning these therapeutic effects. Overall, this research lays the groundwork for leveraging NSC-derived exosomes as a complementary adjunct to NSC transplantation in clinical settings post-stroke.
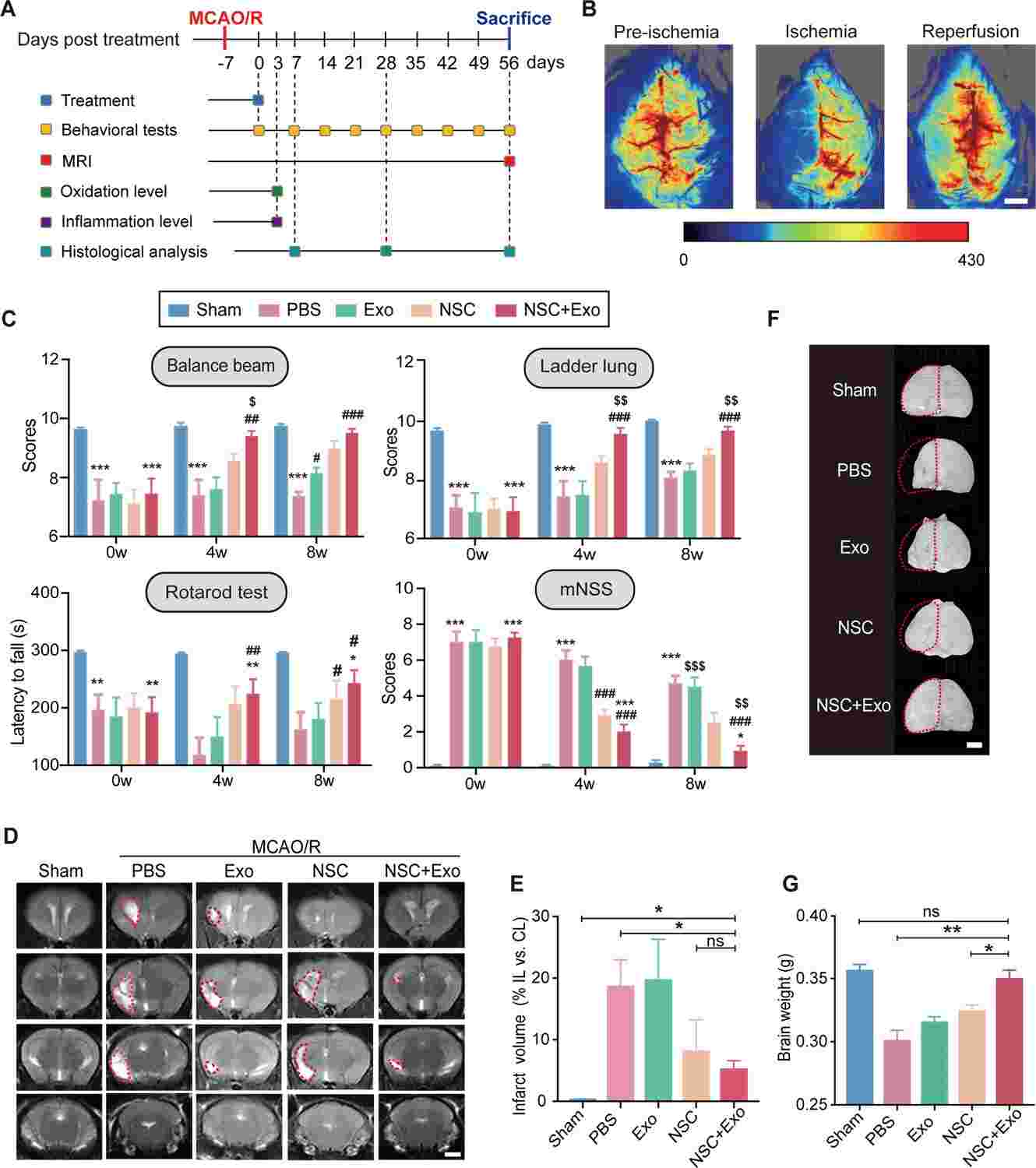 Fig.2 Neural stem cell (NSC)-derived exosomes enhanced the therapeutic effects of NSCs on motor impairment and brain infarction after stroke.
Fig.2 Neural stem cell (NSC)-derived exosomes enhanced the therapeutic effects of NSCs on motor impairment and brain infarction after stroke.Related References
- Tang Y, Yu P, Cheng L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017;8(10):e3108.
- Takahashi T. Multiple roles for cholinergic signaling from the perspective of stem cell function. Int J Mol Sci. 2021;22(2):666.
- Vieira MS, Santos AK, Vasconcellos R, et al. Neural stem cell differentiation into mature neurons: Mechanisms of regulation and biotechnological applications. Biotechnol Adv. 2018;36(7):1946-1970.
- Tuazon JP, Castelli V, Lee JY, et al. Neural stem cells. Adv Exp Med Biol. 2019;1201:79-91.

